Abstract
The central nervous system (CNS) is susceptible to bacterial, viral, and fungal infections. Suppression of the immune system by human immunodeficiency virus (HIV) infection or immunosuppressive therapy after transplantation increases susceptibility to CNS infection and modifies the presentation, diagnosis, and recommended treatment of various CNS infections. This chapter discusses how suppression of the host immune status modifies the presentation, diagnosis, and treatment of selected CNS infections.
IMMUNOSUPPRESSION ASSOCIATED WITH HIV INFECTION
Neurologic illness occurs in 40% to 60% of HIV-infected people.87 Infection of the CNS may occur during any stage of HIV infection, but opportunistic infection occurs only during late-stage infection, when the CD4 count falls below 200 cells/dL.114 Opportunistic infection may affect the brain or spinal cord, and onset may be acute, subacute, or chronic. The most common opportunistic CNS infections and neoplasms are: Toxoplasma encephalitis (TE), cryptococcal meningitis, primary CNS lymphoma (PCNSL), progressive multifocal leukoencephalopathy (PML), AIDS dementia complex (ADC, also known as HIV-associated dementia), and cytomegalovirus (CMV) encephalitis.10 Focal brain lesions occur in up to 17% of people with AIDS and are most often caused by TE, PML, or PCNSL.6 Since the introduction of potent antiretroviral therapy (previously called highly active antiretroviral therapy, or HAART), the incidence of TE and PCNSL has decreased, whereas the incidence of PML has increased.5, 21, 98, 107
IMMUNOSUPPRESSION ASSOCIATED WITH TRANSPLANTATION
Neurologic complications occur in 30% to 60% of people receiving solid organ transplantation and in 12% to 70% of people receiving bone marrow transplantation (BMT). Complications include infection of the CNS, encephalopathy, seizure, stroke, and peripheral neuropathy.47, 61, 109 Infection of the CNS occurs in 5% to 10% of transplant patients and most often manifests as brain abscess, encephalitis, or meningitis.42 Aspergillus fumigatus, Listeria monocytogenes, and Cryptococcus neoformans are the most common causes of CNS infections in post-transplant patients.
Immunosuppressive therapy reduces cell-mediated immunity to prevent rejection of transplant and graft versus host disease (GVHD), but this immunosuppression increases risk of infection by fungi, viruses (especially herpesviruses), bacteria, and parasites. In addition, some immunosuppressive agents, notably cyclosporine and tacrolimus (FK-506), can cause CNS leukoencephalopathy or peripheral neuropathy that can mimic CNS infection.57, 125 Patients who receive autologous BMT (stem cells from patient’s bone marrow or peripheral blood) are much less likely to develop CNS infection than patients who receive allogeneic BMT (stem cells from an HLA-matched donor).61
Susceptibility to CNS infection after transplantation changes over time.42, 109 During the initial month, CNS infection is most often caused by common bacterial pathogens or opportunistic pathogens present in either the transplant environment (e.g., Aspergillus species), or host (e.g., Mycobacterium tuberculosis). At 1 to 6 months, immunosuppression is at its highest, resulting in increased susceptibility to CNS infection by the herpesviruses, especially CMV and Epstein-Barr virus (EBV), fungi, and atypical bacteria. Finally, after 6 months, reduction of immunosuppression is accompanied by decreased susceptibility to CNS infection. If a patient requires continued high levels of immunosuppression because of rejection of graft or GVHD, increased susceptibility to opportunistic CNS infection will persist. Most cases of PML and cryptococcal meningitis occur 6 months post-transplantation.
CLINICAL MANIFESTATIONS
Most opportunistic CNS infections and neoplasms are associated with headache, fever, meningismus, altered level of consciousness, or focal neurologic deficit. The presence of one or more of these symptoms should alert the medical care provider to the possibility of CNS infection. In people with AIDS, a normal (“non-focal”) neurologic examination can be present with PML, cryptococcal meningitis, HIV-associated dementia, or CMVE. Bacterial or viral meningitis can occur at any stage of HIV infection and is typically accompanied by fever.
After transplantation, immunosuppressive therapy reduces the inflammatory response to infection, thus blunting typical symptoms of CNS infection.71 Unlike immunocompetent patients with CNS abscess, post-transplant patients usually manifest with only headache, altered mental status, or fever, without focal neurologic deficits.42, 65 Focal neurologic deficits, when present, are most often seen with toxoplasmosis, aspergillosis, PML, or other fungal abscesses. Clinical and radiologic features of a CNS lesion may distinguish between the various opportunistic infections and neoplasms (Tables 1 and 2).
Table 1.
DISTINGUISHING FEATURES OF NEUROLOGIC DISORDERS ASSOCIATED WITH HIV INFECTION
| Etiology | CD4 Count (cells/mm3) | Common Clinical Features | Neuroimaging Findings by MRI or CT Scanning | Diagnosis |
|---|---|---|---|---|
| Fungal Infections | ||||
| Cryptococcal meningitis | <200 | Fever; bilateral headache; altered mental status; meningeal signs (photophobia, nuchal rigidity) | Normal; meningeal enhancement or enhancing lesion (cryptococcoma) may be present | Presence of CrAg in serum and CSF; positive CSF culture of C. neoformans; positive CSF india ink test |
| Parasitic Infections | ||||
| Toxoplasma encephalitis | <200 | Fever; unilateral or bilateral headache; altered mental status; seizures; focal neurologic deficit: hemiparesis, ataxia, facial weakness | Solitary or multiple ring-enhancing lesions located in the basal ganglia, deep white matter or hemispheric grey-white junction; MRI more sensitive than CT scanning and may detect more lesions | Serum anti-Toxoplasma IgG antibody usually present; definitive diagnosis by identification of trophozoiites on brain biopsy, but presumptive diagnosis by radiologic and clinical improvement after 10–14 days of anti-Toxoplasma therapy |
| Viral Infections | ||||
| Progressive multifocal leukoencephalopathy (JC Virus) | <100 | Unilateral or bilateral headache; visual field deficit; subacute onset of hemiparesis or other focal neurologic deficits; seizures | Solitary or multiple nonenhancing white matter lesions on CT scanning or MRI; lesions most often in parieto-occipital region; on MRI, lesions hypointense on T1-weighted imaging and hyperintense on T2-weighted imaging | CSF PCR for JC virus is sensitive and specific; brain biopsy |
| Primary CNS Lymphoma (Epstein-Barr Virus) | <100 | Unilateral or bilateral headache; focal neurologic deficit; seizures | Solitary or multiple ring- or homogeneously enhancing lesions; may see nodular ventricular lesions or lesions that cross the midline | CSF PCR for Epstein-Barr virus is sensitive and specific; brain biopsy |
| AIDS dementia complex | <200 | Impaired memory and concentration; psychomotor slowing; apathy or withdrawal | Atrophy; on CT scanning diffuse white matter hypodensity; on MRI white matter hyperintense on T2-weighted imaging; no contrast-enhancing lesions | Clinical diagnosis; CSF β2 microglobulin >3.8 mg/l specific, but not sensitive |
| Bacterial Infections | ||||
| M. tuberculosis of CNS | Any | Insidious onset of headeache, fever, and malaise, followed by meningismus, cranial nerve deficits, and mental status changes. Involvement of intracranial arteries may result in stroke. | Ring-enhancing or nonenhancing lesions, or normal. Patients with focal lesions without focal neurologic signs are more likely to have TE than CNS TB. HIV-infected people more often have intracerebral mass lesions. | CSF notable for lymphocytic pleocytosis, hypoglycorrhachia, increased protein, or elevated ADA. AFB smear positive in 37% of initial CSF exam, but 87% if four serial CSF samples examined. Anergy to tuberculin skin testing is common |
Table 2.
DISTINGUISHING FEATURES OF SELECT CNS INFECTIONS ASSOCIATED WITH TRANSPLANTATION
| Etiology | Period of Greatest Risk | Common Clinical Features | Neuroimaging Findings on MRI or CT Scanning | Diagnosis |
|---|---|---|---|---|
| Fungal Infections | ||||
| Aspergillus fumigatus | < 1 month | Usually accompanied by pulmonary or gastrointestinal disease | Multiple nonenhancing hypodense lesions in hemispheric grey-white junction or basal ganglia | Identification of branching, often septate hyphae, or positive culture for A. fumigatus in brain tissue or from other site (e.g., lungs) with characteristic brain imaging findings |
| Candida species | Usually accompanied by disseminated disease and fungemia | Often normal | Identification of Candida species in brain tissue or CSF | |
| Cryptococcus neoformans | > 6 months | Fever; headache; altered mental status | Normal; meningeal enhancement or enhancing lesion(cryptococcoma) may be present | Positive CSF culture of C. neoformans; (CrAg) in CSF |
| Parasitic Infections | ||||
| Toxoplasma encephalitis | Fever; headache; altered mental status; seizures; focal neurologic deficit: hemiparesis, ataxia, facial weakness | Solitary or multiple ring-enhancing lesions located in the basal ganglia, deep white matter or hemispheric grey-white junction | Serum anti-Toxoplasma IgG antibody usually present; definitive diagnosis by identificatior of trophozoiites on brain biopsy | |
| Viral Infections | ||||
| CMV | 1–6 months | Mental status changes, psychomotor slowing, cranial nerve palsies, retinitis | Nodular, enhancing ventriculoencephalitis | CSF PCR for CMV sensitive and specific; brain biopsy |
| HHV-6 | < 3 months | Mental status changes, seizures, cranial nerve deficits | Focal or diffuse encephalitis | Primary infection is distinguished from reactivation by absence of serum IgG; viremi (either by blood culture or PCR of plasma, serum or CSF) diagnostic of active infection. |
| VZV | < 6 months | Disseminated infection; Zoster; encephalitis: may present without cutaneous involvement; headache, confusion and somnolence, | May be a mixture of ischemic or hemorrhagic infarcts and demyelinating lesions, often at grey-white matter junction | CSF PCR for VZV is sensitive and specific; brain biopsy |
| (PTLD) (Epstein-Barr Virus) | > 6 months | Mental status change, hemiparesis, or other focal neurologic deficit | Focal lesion with variable enhancement; may have associated hemorrhage or leptomeningeal spread | CSF PCR for Epstein-Barr virus sensitive and specific; brain biopsy More than 500 copies of EBV per 105 lymphocytes correlates with diagnosis |
| Progressive multifocal leukoencephalopathy (JC Virus) | > 6 months | Mental status changes, visual field deficits, focal neurologic deficit | Solitary or multiple nonenhancing white matter lesions on CT scanning or MRI lesions most often in parieto-occipital region | CSF PCR for JC virus is sensitive and specific; brain biopsy |
| Bacterial Infections | ||||
| M. tuberculosis of CNS | < 1 month or > 6 months | Headache, fever, and malaise, meningismus, cranial nerve deficits, and mental status changes. | Ring-enhancing or nonenhancing lesions. | Lymphocytic pleocytosis, hypoglycorrhachia increased protein, or elevated ADA. AFB smear positive in 37% of initial CSF exam, but 87% if four serial samples examined. |
DIAGNOSIS
Evaluation of potential CNS infection should include neuroimaging with computerized tomography (CT) scanning or magnetic resonance imaging (MRI) with and without administration of an intravenous contrast agent. Characteristic lesion location and contrast enhancement pattern can determine the most likely infection or neoplasm (Table 3).
Table 3.
ETIOLOGIES OF CNS ABNORMALITIES NOTED WITH NEUROIMAGING STUDIES
| Findings on Neuroimaging | Most Common Etiologies During HIV Infection | Less Common Etiologies | Most Common Etiologies After Transplantation | Less Common Etiologies |
|---|---|---|---|---|
| Mass lesion with ring enhancement (enhancement may be absent in post-transplant patient) |
Toxoplasma gondii Epstein-Barr virus (primary CNS lymphoma) Mycobacteria tuberculosis |
Cryptococcus neoformans Kaposi’s sarcoma Metastatic or primary malignancy Bacterial or fungal abscess |
Aspergillus sp. T. gondii Nocardia sp. Bacterial abscess |
Coccidiodes immitis Histoplasma capsulatum Mucor sp. Mycobacteria tuberculosis |
| Non-enhancing lesion in white matter | PML | Multiple sclerosis Stroke |
Aspergillus sp. Cyclosporine or FK-506 PML |
Cranial irradiation Multiple sclerosis Stroke |
| Diffuse atrophy with non-enhancing white matter | AIDS dementia complex (also may see late enhancement of basal ganglia) | PML | Cranial irradiation | |
| Meningeal enhancement |
C. neoformans HSV VZV Bacteria |
Atypical bacteria |
L. monocytogenes C. neoformans |
M. tuberculosis C. immitis |
| Encephalitis | Typical viral pathogens | HHV-6 | EBV VZV |
HHV-6 CMV |
| Ventricular enhancement | CMV | Primary CNS lymphoma | CMV | |
| Normal | C. neoformans | Candida species |
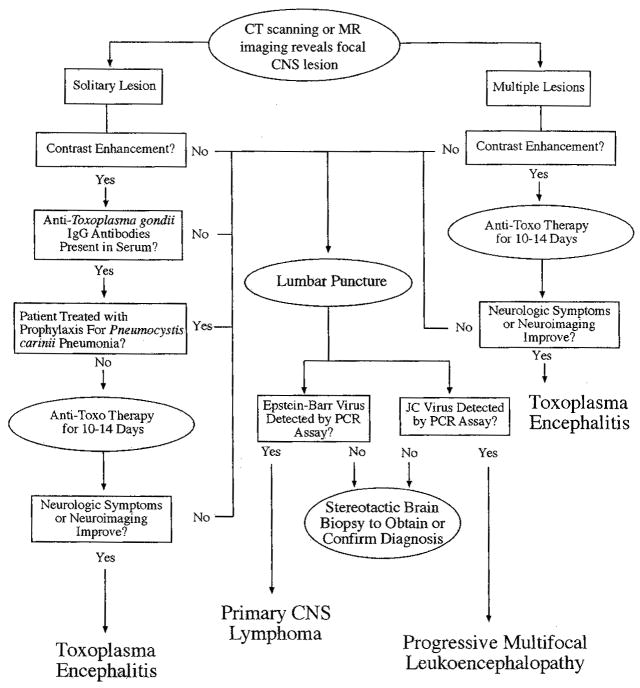
Evaluation of a solitary ring-enhancing CNS mass lesion in a patient with AIDS should be guided: 1) CD4 count; 2) serologic status to T. gondii and C. neoformans; 3) findings on neurologic examination, and; 4) presence or absence of headache or fever. Lumbar puncture may be useful for differentiating between TE and PCNSL (Fig. 1). If the CD4 count is greater than 200 cells/mL, opportunistic CNS infection or neoplasm is unlikely, and the differential diagnosis should include bacterial, fungal, or mycobacterial abcesses, syphilitic gumma, and stroke.
Figure 1.
Algorithm for evaluation of central nervous system lesions in the HIV-infected patient.
Evaluation of a CNS mass lesion in a post-transplant patient should be guided by: 1) time since transplantation; 2) immunosuppressive therapy being received; 3) serologic status to T. gondii and C. neoformans; 4) concomitant pulmonary or gastrointestinal (GI) symptoms; and, 5) findings on chest radiograph or CT scanning. Pulmonary infection usually precedes or accompanies CNS infection by A. fumigatus, C. neoformans, Nocardia asteroides, M. tuberculosis, and the endemic mycoses.65, 109 Type of transplantation received is associated with increased risk for certain CNS infections (Table 4).
Table 4.
PATHOGENS ASSOCIATED WITH TYPE OF TRANSPLANTATION
| Type of Transplantation | Associated Pathogens | Comments |
|---|---|---|
| Heart |
T. gondi EBV (PTLD) |
At higher risk of perioperative cerebrovascular events |
| Lung | PTLD Aspergillus sp. |
PTLD more common in patients with primary EBV infection |
| Liver | Enteric organisms L. monocytogenes C. neoformans |
During first month after transplantation In the presence of chronic active hepatitis Also at higher risk of central pontine myelinolysis |
| Kidney | Gram-negative organisms CMV, EBV |
During first month after transplantation Encephalopathy may be seen with acute rejection of transplant |
| Bone marrow | Herpes viruses | Also most common cause of infection in organ transplant recipients |
INVASIVE DIAGNOSTIC TESTS
Lumbar puncture (LP) should be considered in any person presenting with new-onset headache, fever, or mental status change. If the patient is obtunded or comatose, or a focal deficit neurologic deficit is present, neuroimaging should be performed before LP. If a space-occupying CNS lesion is present in the posterior fossa or causes midline shift, LP should be avoided because CNS herniation could result.2 Cerebrospinal fluid (CSF) testing should include cell count with differential, glucose, protein, bacterial culture, and VDRL. Other tests that should be considered include fungal cultures, cryptococcal polysaccharide capsular antigen (CrAg), and polymerase chain reaction (PCR) assays. If symptoms or brain imaging are consistent with herpesvirus infection or PML, PCR assay for the associated viral pathogen should be requested. The sensitivity and specificity of most PCR assays are high (Table 5). In people with AIDS, an elevated level of β2 microglobulin in the CSF (greater than 3.8 mg/l) is specific, but not sensitive for the diagnosis of HIV-associated dementia.20, 99 In post-transplant patients, presumptive diagnosis of CNS mass lesion may be made through pathogen identification in culture or biopsy from abnormal pulmonary or gastrointestinal tissue.23, 65 Unfortunately, PCR assays of the CSF are not yet adequate for diagnosis of fungal or M. tuberculosis infection of the CNS.
Table 5.
SENSITIVITY AND SPECIFICITY OF PCR ASSAYS FOR SELECTED OPPORTUNISTIC CNS INFECTIONS
| Pathogen | Associated Syndrome | Sensitivity (%) | Specificity (%) |
|---|---|---|---|
| EBV | Primary CNS lymphoma | 97 | 100 |
| JC Virus | Progressive multifocal leukoencephalopathy | 74–92 | 92–96 |
| CMV | CMV ventriculitis and polyradiculopathy | 80–100 | 75–100 |
| Varicella-Zoster Virus | VZV encephalitis and Varicella-Zoster | Unknown | 100 |
| HSV-1 | HSV encephalitis | >95 | 100 |
| Mycobacterium tuberculosis | CNS tuberculosis | 48–100 | 100 |
BRAIN BIOPSY
The most common causes of focal CNS lesions in people with AIDS are toxoplasma encephalitis and PCNSL. The differential diagnosis also includes fungal or atypical bacterial abscess, cryptococcoma, syphilitic gumma, tuberculoma, cerebrovascular disease, and neoplasms other than PCNSL.58 Stereotactic brain biopsy (SBB) of a CNS lesion may be necessary for certain clinical scenarios: if a solitary CNS lesion is accompanied by negative toxoplasmosis serology; if a contrast-enhancing lesion is atypical for TE or does not respond to anti-toxoplasma treatment; if a new lesion develops during anti-toxoplasma maintenance treatment; or if histopathologic diagnosis is required for entry into an experimental treatment protocol.28, 31, 72 If CNS herniation is imminent, open biopsy and decompression should be considered, unless the patient is terminal or has previously requested no intervention. Stereotactic brain biopsy provides a diagnosis for 88% to 98% of contrast-enhancing lesions, and 67% of nonenhancing lesions.28, 58 Studies of how SBB affects treatment and outcome of CNS disease have demonstrated that biopsy results can influence treatment decisions that can increase life expectancy.28, 70, 72
Complications of SBB occur in 3% to 12% of patients with HIV infection and include hemorrhage, neurologic deficits, seizures, and infection. Mortality occurred in 2% to 8% of patients.7, 28, 53, 58, 72 These rates are slightly higher than rates associated with SBB in people without HIV infection.16, 80, 94, 133
In post-transplant patients, most focal CNS infections are caused by Aspergillus sp., Candida sp., or other fungal species. One study of 58 cases of brain abscess after bone marrow transplantation noted that only nine (16%) underwent brain biopsy. All nine biopsies provided the correct diagnosis.65 Of note, of the 29 cases of Aspergillus brain abscess confirmed by autopsy, 27 (87%) had concomitant pulmonary aspergillosis and 10 (33%) had Aspergillus identified on culture of lung tissue, sputum, or chest-tube drainage. In addition, of 19 patients with candidal brain abscess, 12 (63%) were fungemic at the time of diagnosis. In summary, identification of the causative organism of brain abscess in post-transplant patients can often be obtained by fungal blood culture or histopathologic examination of secretions or tissue from the respiratory tract, without SBB.
DIAGNOSIS AND TREATMENT OF SPECIFIC INFECTIONS
Identical pathogens can cause different symptoms and variations in recommended treatment of CNS infection, according to whether the patient is immunocompromised because of AIDS or transplantation (Table 6). This section describes these variations associated with specific pathogens.
Table 6.
MEDICAL THERAPY OF SELECTED CNS INFECTIONS
| Infection | Therapy | Dose and Duration | Comment |
|---|---|---|---|
| Cryptococcal meningitis | Amphotericin B and Flucytosine (5-FC) then Fluconazole | 0.6–0.8 mg/kg/day IV for 14 days, or until headache, fever, nausea, and vomiting resolve 75–100 mg/kg/day PO 400 mg/day until CSF culture negative, then decrease to200 mg/day and continue for life |
In patients with AIDS, fluconazole associated with delayed sterilization of CSF and more early deaths. If intracranial pressure increases, repeat lumbar punctures one to four times daily to remove15–30 mL of CSF, until opening pressure consistently normal. Flucytosine (5-FC) should be used concurrently with amphotericin B; may cause marrow suppression or leukopenia Lumbar puncture should be repeated after amphotericin B, then every 2 to 4 weeks, or sooner if clinical deterioration occurs. Once CSF culture is negative, fluconazole should be started. CSF CrAg can persist positive even if culture is negative, and should not guide therapy. If CSF CrAg titer rises above initial titer, repeat treatment with amphotericin Unlike patients with AIDS, who require prophylactic treatment after cryptococcal infection, post-transplant patients require treatment for only 2–6 weeks after clearance of CrAg |
| Toxoplasma encephalitis | Pyrimethamine and Folinic acid and Sulfadiazine or Clindamycin | 100–200 mg load, then 75–100 mg/day PO 10–50 mg/day PO 4–8 g/day (100 mg/kg/day) PO divided into four doses 600–900 mg PO/IV qid. |
Alternatives to sulfadiazine: 1) Atovaquone: 750 mg PO qid; 2) Clarithromycin: 1 g PO bid; 3) Azithromycin: 1 g load, then 500 mg/day. Consider sulfa desensitization for sulfa-allergic people Lifetime maintenance dose required for patients with AIDS: 1) Pyrimethamine: 25–50 mg/day; 2) Folinic acid: 10–50 mg/day; 3) Sulfadiazine: 1 g tid-qid, or Clindamycin: 300–450 mg tid-qid Post-transplant patients receive prophylaxis with trimethoprim/sulfamethoxazole for 6 months |
| EBV-related neoplasms (Primary CNS lymphoma and post-transplant lymphoproliferative disorder) | Radiotherapy Chemotherapy |
Whole-brain irradiation with 4,000 cGy with a “boost” of 1,000 to 2,000 cGy focused on the tumor bed Methotrexate (intravenous and intrathecal), thiotepa, and procarbazine. |
Radiation or combined modality treatments prolong life by several months (27 days versus 119 days mean survival in persons receiving radiation therapy) Steroids (dexamethasone) may reduce edema associated with tumor. Mortality rate of PTLD is 50%. Reduced immunosuppressive therapy may improve outcome |
| Progressive multifocal leukoencephalopathy | Antiretroviral therapy | Potent antiretroviral therapy | No effective treatments. Anecdotal reports of efficacy of antiretroviral therapy; spontaneous remissions and prolonged survival in 5%–10%, but average life expectancy is usually months |
| (JC Virus) | Cytarabine | 2 mg/kg/d for 5 days; may repeat single dose in six weeks | Cytarabine not effective in patients with AIDS, but may be effective in post-transplant patients. Reduction of immunosuppression can produce resolution of infection |
| Cytomegalovirus | Gancyclovir or Foscarnet | 5 mg/kg BID or TID for 2–4 weeks (induction) then5 mg/kg/day 5–7 x/week (maintenance) 60 mg/kg q8H for 2–3 weeks(induction), then90–120 mg/kg/day IV6–7 x/week (maintenance) |
There may be synergy if gancyclovir and foscarnet are used together. Resistance to gancyclovir has occurred in patients with polyradiculopath Poor prognosis associated with previous treatment for CMV retinitis, Karnofsky score less than 70, persistently positive CSF CMV PCR, persistent hypoglycorrhachia. Average life expectancy for CMVE or polyradiculopathy is weeks to months Recommended treatment for severely affected patients: induction with gancyclovir and foscarnet, then monotherapy after 2 weeks of therapy if there is improvement in symptoms or lower quantitative CSF CMV PCR. Improvement of symptoms may take weeks to months |
| Human herpesvirus-6 | Gancyclovir or Foscarnet | Dosing same as for CMV infection | Acyclovir not effective (similar to CMV, HHV-6 lacks thymidine kinase) |
| Varicella-zoster virus | Acyclovir Famciclovir Valacyclovir |
10–14 mg/kg/QID 500 mg QID for 7 days 1g QID for 7 days |
Primary infection: disseminated cutaneous or visceral infection, requires treatment with acyclovir VZV seronegative post-transplant patients should receive varicella-zoster immune globulin (VZIG) if exposed to persons with VZV infection |
| Tuberculosis of CNS | Isoniazid (INH) pyridoxine Rifampin Pyrazinamide (PZA) Streptomycin or Ethambutol |
300 mg/day 50 mg/day 600 mg day 20–35 mg/kg/day 1 g/day 15–25 mg/kg/day |
INH, rifampin and PZA are bacteriocidal, and all except rifampin penetrate noninflamed meninges into CSF. Corticosteroids should be considered for very ill patients. Four-drug therapy should be used if patient was previously treated for TB, or comes from area with high prevalence of drug resistance Full therapy for 2 months, then INH and rifampin for 4–16 months. Patients with less than 200 CD4 cells/dL or more than 2 weeks of illness Previous initiating TB therapy has lower survival rates. |
Toxoplasmosis
Reactivation of previously acquired infections is responsible for the majority of opportunistic CNS infections caused by T. gondii. In the United States, 10% to 40% of people with AIDS are latently infected with T. gondii, as determined by presence of serum anti-toxoplasma immunoglobulin G (IgG) antibodies. In France, the seroprevalence of anti-toxoplasma IgG in people with AIDS is 80%. One-third of people with serum anti-toxoplasma IgG antibodies will develop TE. The absence of serum anti-toxoplasma IgG or IgM antibodies does not exclude the diagnosis of TE.93, 111 The incidence of TE is reduced in people who take trimethoprim/sulfamethoxazole (TMP/SMZ) or dapsone/pyrimethamine as prophylaxis against PCP.22, 26
In post-transplant patients, toxoplasmosis presents as either primary infection, when a donor organ containing encysted T. gondii is transplanted into a seronegative recipient, or as reactivation of latent infection.92 CNS toxoplasmosis is typically associated with disseminated infection. Prophylactic treatment with TMP/SMX for 6 months after transplantation reduces the risk of infection by T. gondii, L. monocytogenes, N. asteroides, and Pneumocystis carinii. Patients who are seropositive to T. gondii, and heart transplant recipients are at greater risk of developing infection by T. gondii and typically receive higher dosages of TMP/SMZ.103
Cryptococcal Meningitis
Cryptococcus neoformans is a ubiquitous yeast that causes meningitis in 7% of people with AIDS living in the United States and 30% of those living in Africa.30 In people with cryptococcal meningitis, CrAg is detectable in 99% of serum samples and 91% of CSF samples; therefore, a negative serum CrAg virtually excludes the diagnosis of cryptococcal meningitis.113
In post-transplant patients, the lungs are typically the portal of entry for cryptococcal infection, but pulmonary symptoms may not manifest until after the infection has disseminated. Disseminated infection to the skin, skeletal system, urinary tract, or the CNS is also common.42 The majority of cases of C. neoformans infection occur 6 months or more after transplantation. As opposed to patients with AIDS, who require life-long prophylaxis after treatment for C. neoformans infection, post-transplant patients only require treatment for active infection.74 Prophylactic fluconazole reduces the risk of infection by C. neoformans and Candida species.
Herpesvirus Family
The herpesvirus family includes herpes virus type 1 (HSV-1) and type 2 (HSV-2), CMV, EBV, varicella-zoster virus (VZV), and human herpesviruses 6 (HHV-6) and 8 (HHV-8). All these viruses may persist in the human host, in either ganglia (HSV-1 and -2, VZV) or in lymphocytes (CMV, EBV, HHV-6, and -8). Herpesviruses are the most frequent causes of infections in the post-transplant patient. Symptomatic infection can occur with primary infection or reactivation of latent infection. Prophylactic treatment with acyclovir reduces the risk of infection by HSV, VZV, CMV, and EBV.46, 136
Herpes Simplex Viruses
HSV-1 causes 10% to 20% of CNS encephalitides and has a mortality rate of greater than 70% if untreated.44, 137 HSV-2 causes genital herpes infection and is associated with neonatal encephalitis and recurrent benign lymphocytic meningitis in adults.91, 110, 135
Polymerase chain reaction is the test of choice for diagnosis of her pes simplex infections.83, 131 The sensitivity of PCR decreases if antiviral treatment has been given for more than 1 week.9 In people with AIDS, HSV-1 can present as encephalitis and or disseminated infection. In post-transplant patients, prophylactic antiviral medication is typically prescribed, so HSV infection is uncommon. When HSV encephalitis occurs in a post-transplant patient, the clinician should be alert for acyclovir-resistant HSV.
Epstein-Barr Virus
In the immunocompetent host, EBV is associated with a variety of neurologic syndromes, including mononucleosis, encephalitis, aseptic meningitis, Guillain-Barré syndrome, and Bell’s palsy.63 In post-transplant patients and in people with AIDS, EBV infection can be a primary infection or reactivation of latent infection. The clinical presentation of primary infection is similar to that of infectious mononucleosis, with pharyngitis, fever, lymphadenopathy, and hepatosplenomegaly.
In people with AIDS, EBV is associated with nearly 100% of PCNSL.8, 33, 67 Primary CNS lymphomas represent reactivation of latent infection. The presence of serum antibodies against EBV does not correlate with an increased incidence of PCNSL.29
In post-transplant patients, EBV is associated with meningoencephalitis and an abnormal proliferation of lymphoid cells known as post-transplant lymphoproliferative disorders (PTLD).8, 11, 49, 67 PTLD occurs in less than 1% of renal transplant recipients, 1% to 2% of bone marrow transplant recipients, 2% to 4% of liver allograft recipients, and up to 10% of heart–lung transplant recipients. Twenty-eight percent of patients with PTLD have CNS involvement, typically presenting as mental status change, hemiparesis, or other focal neurologic deficit. The incidence of PTLD is highest in the first year after transplantation and most often occurs in patients who were EBV-seropositive before transplantation. Diagnosis of PTLD is confirmed by demonstration of abnormal lymphoid proliferation in biopsy material or body fluid. The presence of serum EBV DNA correlates with an increased risk of development of PTLD.88
Cytomegalovirus
In the immunocompetent host, CMV infection can be asymptomatic or can present as a mononucleosis-like syndrome or as Guillain-Barré syndrome.38, 132 In people with AIDS, CMV can cause polyradiculitis, myelitis, encephalitis, or multifocal neuritis.56, 69, 77 These complications are uncommon in an era of potent antiretroviral therapy. As opposed to the lymphocytic pleocytosis seen with other viral infections of the CNS, CMV infection produces a polymorphonuclear pleocytosis, particularly in individuals with polyradiculitis.32, 33, 60
In the post-transplant patient, CMV is an uncommon cause of encephalitis or Guillain-Barré syndrome but is often associated with pulmonary infection and graft rejection.43, 62 Higher CMV viral loads in blood of solid organ and bone marrow transplant recipients are associated with higher likelihood of CMV-related diseases.62
Varicella-Zoster Virus
Varicella-zoster virus is the causative agent of varicella (chickenpox) and herpes zoster (shingles). Other CNS complications associated with VZV infection include post-varicella cerebellitis, meningoencephalitis (including the Ramsay Hunt syndrome), vasculopathy, and acute aseptic meningitis.4, 41, 51, 73 In people with HIV infection, CNS complications of VZV infection, most notably herpes zoster, occur more frequently than in the general population.130
In the post-transplant patient, primary infection with VZV can rapidly disseminate to multiple organs, including the CNS, and therefore requires emergency treatment when identified. In one study, 5.5% of bone marrow transplant recipients developed VZV infection during a 10-year period: 62% had zoster, and 32% had disseminated, visceral, or CNS infection.68 Those patients who developed disseminated, visceral, or CNS infection did so within 7 months of transplantation. Compared with immunocompetent people, post-transplant patients with zoster are more likely to develop post-herpetic neuralgia.120
Human Herpesvirus-6
Human herpesvirus-6 (HHV-6) is the causative agent of roseola infantum (exanthem subitum) and has been associated with subacute leukoencephalitis, focal encephalitis, chronic myelopathy, and febrile convulsions.27, 79, 96, 100, 139 In patients with AIDS, HHV-6 can cause pneumonitis and encephalitis. In addition, HHV-6 can coinfect HIV-infected cells and may be a cofactor in the acceleration of HIV infection.78, 95
Infection by HHV-6 occurs in 38% to 60% of bone marrow transplant patients and 31% to 55% of solid organ transplant patients. In bone marrow transplant patients as well as in organ transplant patients, HHV-6 infection has been associated with bone marrow suppression, interstitial pneumonitis, and encephalitis.39, 124 Reactivation of latent infection is responsible for most cases of infection, but transmission of virus from donor tissue can occur.
JC Virus
The majority of people in the United States have serum antibodies against JC virus, a papova virus that causes PML.29 PML is a subacute progressive demyelinating disease of the CNS that most commonly occurs in people with AIDS, but can also occur in post-transplant patients. The clinical presentation of PML in post-transplant patients is similar to that of patients with AIDS. Unlike other viral infections of the CNS, PML usually presents many months or years after transplantation. Reactivation of latent JC virus infection causes PML and can be detected by PCR assay in the CSF of 92% of patients with PML.102 Detection of JCV DNA in the urine or blood is not predictive of PML.81 One study of patients with AIDS showed a negative correlation between CSF JCV DNA concentration and survival. Those patients with PML and greater than 5log10 JCV equivalents/mL CSF died within 8 months of diagnosis compared with those patients with less than 5log10 JCV equivalents/mL CSF who survived at least 19 months.128
Mycobacterium Tuberculosis
Mycobacterium tuberculosis can cause meningitis, tuberculoma, brain abscess, myelopathy, or radiculopathy. Tuberculosis of the CNS can occur at any stage of HIV infection, and is often intracerebral and accompanied by anergy to skin testing.17, 50 Patients with CNS tuberculosis typically have an insidious onset of headache, fever, and malaise, followed by meningismus, cranial nerve deficits, and mental status changes.14 People with AIDS who have a focal CNS lesion without focal neurologic signs are more likely to have TE than CNS tuberculosis.122 Stroke may occur when M. tuberculosis infects the intracranial arteries, most commonly in the anterior circulation.85
In post-transplant patients, the incidence of M. tuberculosis infection is uncommon but is higher than in the general population.117 Infection of the CNS may present as meningitis, with headache, fever, and cranial nerve deficits, or less commonly as parenchymal infection.25 Most cases of M. tuberculosis infection represent reactivation of latent disease and occur more than 6 months after transplantation. If infection occurs within weeks or months of transplantation, primary infection or active disease at time of transplantation should be suspected.104, 117
SUMMARY
The CNS is susceptible to bacterial, viral, and fungal infections. Examination of the level and type of immunosuppression, in addition to the clinical and radiologic findings at the time of diagnosis, can help the clinician determine the most likely etiology of infection. Treatment of active infection, as well as prophylactic treatment to prevent active infection, varies with type of immunosuppression.
References
- 1.Evaluation and management of intracranial mass lesions in AIDS. Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 1998;50:21–26. doi: 10.1212/wnl.50.1.21. [DOI] [PubMed] [Google Scholar]
- 2.Practice parameters: Lumbar puncture (summary statement). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 1993;43:625–627. [PubMed] [Google Scholar]
- 3.Adair JC, Call GK, O’Connell JB, Baringer JR. Cerebrovascular syndromes following cardiac transplantation. Neurology. 1992;42:819–823. doi: 10.1212/wnl.42.4.819. [DOI] [PubMed] [Google Scholar]
- 4.Amlie-Lefond C, Kleinschmidt-DeMasters BK, Mahalingam R, et al. The vasculopathy of varicella-zoster virus encephalitis. Ann Neurol. 1995;37:784–790. doi: 10.1002/ana.410370612. [DOI] [PubMed] [Google Scholar]
- 5.Ammassari A, Cingolani A, Pezzotti P, et al. AIDS-related focal brain lesions in the era of highly active antiretroviral therapy. Neurology. 2000;55:1194–1200. doi: 10.1212/wnl.55.8.1194. [DOI] [PubMed] [Google Scholar]
- 6.Anders KH, Guerra WF, Tomiyasu U, et al. The neuropathology of AIDS. UCLA experience and review. Am J Pathol. 1986;124:537–558. [PMC free article] [PubMed] [Google Scholar]
- 7.Antinori A, Ammassari A, De Luca A, et al. Diagnosis of AIDS-related focal brain lesions: A decision-making analysis based on clinical and neuroradiologic characteristics combined with polymerase chain reaction assays in CSF. Neurology. 1997;48:687–694. doi: 10.1212/wnl.48.3.687. [DOI] [PubMed] [Google Scholar]
- 8.Arribas JR, Clifford DB, Fichtenbaum CJ, et al. Detection of Epstein-Barr virus DNA in cerebrospinal fluid for diagnosis of AIDS-related central nervous system lymphoma. J Clin Microbiol. 1995;33:1580–1583. doi: 10.1128/jcm.33.6.1580-1583.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aurelius E, Johansson B, Skoldenberg B, et al. Rapid diagnosis of herpes simplex encephalitis by nested polymerase chain reaction assay of cerebrospinal fluid. Lancet. 1991;337:189–192. doi: 10.1016/0140-6736(91)92155-u. [DOI] [PubMed] [Google Scholar]
- 10.Bacellar H, Munoz A, Miller EN, et al. Temporal trends in the incidence of HIV-1-related neurologic diseases: Multicenter AIDS Cohort Study, 1985–1992. Neurology. 1994;44:1892–1900. doi: 10.1212/wnl.44.10.1892. [DOI] [PubMed] [Google Scholar]
- 11.Basgoz N, Preiksaitis JK. Post-transplant lymphoproliferative disorder. Infect Dis Clin North Am. 1995;9:901–923. [PubMed] [Google Scholar]
- 12.Bass JB, Jr, Farer LS, Hopewell PC, et al. Treatment of tuberculosis and tuberculosis infection in adults and children. American Thoracic Society and The Centers for Disease Control and Prevention. Am J Respir Crit Care Med. 1994;149:1359–1374. doi: 10.1164/ajrccm.149.5.8173779. [DOI] [PubMed] [Google Scholar]
- 13.Baumgartner JE, Rachlin JR, Beckstead JH, et al. Primary central nervous system lymphomas: Natural history and response to radiation therapy in 55 patients with acquired immunodeficiency syndrome. J Neurosurg. 1990;73:206–211. doi: 10.3171/jns.1990.73.2.0206. [DOI] [PubMed] [Google Scholar]
- 14.Berenguer J, Moreno S, Laguna F, et al. Tuberculous meningitis in patients infected with the human immunodeficiency virus. N Engl J Med. 1992;326:668–672. doi: 10.1056/NEJM199203053261004. [DOI] [PubMed] [Google Scholar]
- 15.Berger JR, Nath A, Greenberg RN, et al. Cerebrovascular changes in the basal ganglia with HIV dementia. Neurology. 2000;54:921–926. doi: 10.1212/wnl.54.4.921. [DOI] [PubMed] [Google Scholar]
- 16.Bernstein M, Parrent AG. Complications of CT-guided stereotactic biopsy of intra-axial brain lesions. J Neurosurg. 1994;81:165–168. doi: 10.3171/jns.1994.81.2.0165. [DOI] [PubMed] [Google Scholar]
- 17.Bishburg E, Sunderam G, Reichman LB, Kapila R. Central nervous system tuberculosis with the acquired immunodeficiency syndrome and its related complex. Ann Intern Med. 1986;105:210–213. doi: 10.7326/0003-4819-105-2-210. [DOI] [PubMed] [Google Scholar]
- 18.Bonington A, Strang JI, Klapper PE, et al. Use of Roche AMPLICOR Mycobacterium tuberculosis PCR in early diagnosis of tuberculous meningitis. J Clin Microbiol. 1998;36:1251–1254. doi: 10.1128/jcm.36.5.1251-1254.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bozzette SA, Larsen RA, Chiu J, et al. A placebo-controlled trial of maintenance therapy with fluconazole after treatment of cryptococcal meningitis in the acquired immunodeficiency syndrome. California Collaborative Treatment Group. N Engl J Med. 1991;324:580–584. doi: 10.1056/NEJM199102283240902. [DOI] [PubMed] [Google Scholar]
- 20.Brew BJ, Bhalla RB, Paul M, et al. Cerebrospinal fluid beta 2-microglobulin in patients with AIDS dementia complex: An expanded series including response to zidovudine treatment. Aids. 1992;6:461–465. [PubMed] [Google Scholar]
- 21.Brodt HR, Kamps BS, Gute P, et al. Changing incidence of AIDS-defining illnesses in the era of antiretroviral combination therapy. Aids. 1997;11:1731–1738. doi: 10.1097/00002030-199714000-00010. [DOI] [PubMed] [Google Scholar]
- 22.Bucher HC, Griffith L, Guyatt GH, Opravil M. Meta-analysis of prophylactic treatments against Pneumocystis carinii pneumonia and toxoplasma encephalitis in HIV-infected patients. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;15:104–114. doi: 10.1097/00042560-199706010-00002. [DOI] [PubMed] [Google Scholar]
- 23.Burgert SJ, Classen DC, Burke JP, Blatter DD. Candidal brain abscess associated with vascular invasion: A devastating complication of vascular catheter-related candidemia. Clin Infect Dis. 1995;21:202–205. doi: 10.1093/clinids/21.1.202. [DOI] [PubMed] [Google Scholar]
- 24.Burke DG, Kalayjian RC, Vann VR, et al. Polymerase chain reaction detection and clinical significance of varicella-zoster virus in cerebrospinal fluid from human immunodeficiency virus-infected patients. J Infect Dis. 1997;176:1080–1084. doi: 10.1086/516516. [DOI] [PubMed] [Google Scholar]
- 25.Campos A, Vaz CP, Campilho F, et al. Central nervous system (CNS) tuberculosis following allogeneic stem cell transplantation. Bone Marrow Transplant. 2000;25:567–569. doi: 10.1038/sj.bmt.1702163. [DOI] [PubMed] [Google Scholar]
- 26.Carr A, Tindall B, Brew BJ, et al. Low-dose trimethoprim-sulfamethoxazole prophylaxis for toxoplasmic encephalitis in patients with AIDS. Ann Intern Med. 1992;117:106–111. doi: 10.7326/0003-4819-117-2-106. [DOI] [PubMed] [Google Scholar]
- 27.Carrigan DR, Harrington D, Knox KK. Subacute leukoencephalitis caused by CNS infection with human herpesvirus-6 manifesting as acute multiple sclerosis. Neurology. 1996;47:145–148. doi: 10.1212/wnl.47.1.145. [DOI] [PubMed] [Google Scholar]
- 28.Chappell ET, Guthrie BL, Orenstein J. The role of stereotactic biopsy in the management of HIV-related focal brain lesions. Neurosurgery. 1992;30:825–829. doi: 10.1227/00006123-199206000-00001. [DOI] [PubMed] [Google Scholar]
- 29.Chesters PM, Heritage J, McCance DJ. Persistence of DNA sequences of BK virus and JC virus in normal human tissues and in diseased tissues. J Infect Dis. 1983;147:676–684. doi: 10.1093/infdis/147.4.676. [DOI] [PubMed] [Google Scholar]
- 30.Chuck SL, Sande MA. Infections with Cryptococcus neoformans in the acquired immunodeficiency syndrome. N Engl J Med. 1989;321:794–799. doi: 10.1056/NEJM198909213211205. [DOI] [PubMed] [Google Scholar]
- 31.Cimino C, Lipton RB, Williams A, et al. The evaluation of patients with human immunodeficiency virus-related disorders and brain mass lesions. Arch Intern Med. 1991;151:1381–1384. [PubMed] [Google Scholar]
- 32.Cinque P, Bossolasco S, Vago L, et al. Varicella-zoster virus (VZV) DNA in cerebrospinal fluid of patients infected with human immunodeficiency virus: VZV disease of the central nervous system or subclinical reactivation of VZV infection? Clin Infect Dis. 1997;25:634–639. doi: 10.1086/513754. [DOI] [PubMed] [Google Scholar]
- 33.Cinque P, Brytting M, Vago L, et al. Epstein-Barr virus DNA in cerebrospinal fluid from patients with AIDS-related primary lymphoma of the central nervous system. Lancet. 1993;342:398–401. doi: 10.1016/0140-6736(93)92814-a. [DOI] [PubMed] [Google Scholar]
- 34.Cinque P, Cleator GM, Weber T, et al. Diagnosis and clinical management of neurological disorders caused by cytomegalovirus in AIDS patients. European Union Concerted Action on Virus Meningitis and Encephalitis. J Neurovirol. 1998;4:120–132. doi: 10.3109/13550289809113490. [DOI] [PubMed] [Google Scholar]
- 35.Clifford DB, Buller RS, Mohammed S, et al. Use of polymerase chain reaction to demonstrate cytomegalovirus DNA in CSF of patients with human immunodeficiency virus infection. Neurology. 1993;43:75–79. doi: 10.1212/wnl.43.1_part_1.75. [DOI] [PubMed] [Google Scholar]
- 36.Cohen BA. Prognosis and response to therapy of cytomegalovirus encephalitis and meningomyelitis in AIDS. Neurology. 1996;46:444–450. doi: 10.1212/wnl.46.2.444. [DOI] [PubMed] [Google Scholar]
- 37.Cohen BA, McArthur JC, Grohman S, et al. Neurologic prognosis of cytomegalovirus polyradiculomyelopathy in AIDS. Neurology. 1993;43:493–499. doi: 10.1212/wnl.43.3_part_1.493. [DOI] [PubMed] [Google Scholar]
- 38.Cohen JI, Corey GR. Cytomegalovirus infection in the normal host. Medicine (Baltimore) 1985;64:100–114. doi: 10.1097/00005792-198503000-00003. [DOI] [PubMed] [Google Scholar]
- 39.Cole PD, Stiles J, Boulad F, et al. Successful treatment of human herpesvirus 6 encephalitis in a bone marrow transplant recipient. Clin Infect Dis. 1998;27:653–654. doi: 10.1086/517145. [DOI] [PubMed] [Google Scholar]
- 40.Coley SC, Jager HR, Szydlo RM, Goldman JM. CT and MRI manifestations of central nervous system infection following allogeneic bone marrow transplantation. Clin Radiol. 1999;54:390–397. doi: 10.1053/crad.1999.0200. [DOI] [PubMed] [Google Scholar]
- 41.Connolly AM, Dodson WE, Prensky AL, Rust RS. Course and outcome of acute cerebellar ataxia. Ann Neurol. 1994;35:673–679. doi: 10.1002/ana.410350607. [DOI] [PubMed] [Google Scholar]
- 42.Conti DJ, Rubin RH. Infection of the central nervous system in organ transplant recipients. Neurol Clin. 1988;6:241–260. [PubMed] [Google Scholar]
- 43.Cordonnier C, Feuilhade F, Vernant JP, et al. Cytomegalovirus encephalitis occurring after bone marrow transplantation. Scand J Haematol. 1983;31:248–252. doi: 10.1111/j.1600-0609.1983.tb00648.x. [DOI] [PubMed] [Google Scholar]
- 44.Corey L, Spear PG. Infections with herpes simplex viruses (2) N Engl J Med. 1986;314:749–757. doi: 10.1056/NEJM198603203141205. [DOI] [PubMed] [Google Scholar]
- 45.Dannemann B, McCutchan JA, Israelski D, et al. Treatment of toxoplasmic encephalitis in patients with AIDS. A randomized trial comparing pyrimethamine plus clindamycin to pyrimethamine plus sulfadiazine. The California Collaborative Treatment Group. Ann Intern Med. 1992;116:33–43. doi: 10.7326/0003-4819-116-1-33. [DOI] [PubMed] [Google Scholar]
- 46.Darenkov IA, Marcarelli MA, Basadonna GP, et al. Reduced incidence of Epstein-Barr virus-associated posttransplant lymphoproliferative disorder using preemptive antiviral therapy. Transplantation. 1997;64:848–852. doi: 10.1097/00007890-199709270-00010. [DOI] [PubMed] [Google Scholar]
- 47.Davis DG, Patchell RA. Neurologic complications of bone marrow transplantation. Neurol Clin. 1988;6:377–387. [PubMed] [Google Scholar]
- 48.De Luca A, Giancola ML, Ammassari A, et al. Cidofovir added to HAART improves virological and clinical outcome in AIDS-associated progressive multifocal leukoencephalopathy. Aids. 2000;14:F117–F121. doi: 10.1097/00002030-200009290-00001. [DOI] [PubMed] [Google Scholar]
- 49.Dellemijn PL, Brandenburg A, Niesters HG, et al. Successful treatment with ganciclovir of presumed Epstein-Barr meningo-encephalitis following bone marrow trans plant. Bone Marrow Transplant. 1995;16:311–312. [PubMed] [Google Scholar]
- 50.Dube MP, Holtom PD, Larsen RA. Tuberculous meningitis in patients with and without human immunodeficiency virus infection. Am J Med. 1992;93:520–524. doi: 10.1016/0002-9343(92)90579-z. [DOI] [PubMed] [Google Scholar]
- 51.Echevarria JM, Casas I, Tenorio A, et al. Detection of varicella-zoster virus-specific DNA sequences in cerebrospinal fluid from patients with acute aseptic meningitis and no cutaneous lesions. J Med Virol. 1994;43:331–335. doi: 10.1002/jmv.1890430403. [DOI] [PubMed] [Google Scholar]
- 52.Emanuel DJ, Lucas KG, Mallory GB, Jr, et al. Treatment of posttransplant lymphoproliferative disease in the central nervous system of a lung transplant recipient using allogeneic leukocytes. Transplantation. 1997;63:1691–1694. doi: 10.1097/00007890-199706150-00027. [DOI] [PubMed] [Google Scholar]
- 53.Feiden W, Bise K, Steude U, et al. The stereotactic biopsy diagnosis of focal intracerebral lesions in AIDS patients. Acta Neurol Scand. 1993;87:228–233. doi: 10.1111/j.1600-0404.1993.tb04107.x. [DOI] [PubMed] [Google Scholar]
- 54.Fong IW, Toma E. The natural history of progressive multifocal leukoencephalopathy in patients with AIDS. Canadian PML Study Group. Clin Infect Dis. 1995;20:1305–1310. doi: 10.1093/clinids/20.5.1305. [DOI] [PubMed] [Google Scholar]
- 55.Forsyth PA, Yahalom J, DeAngelis LM. Combined-modality therapy in the treatment of primary central nervous system lymphoma in AIDS. Neurology. 1994;44:1473–1479. doi: 10.1212/wnl.44.8.1473. [DOI] [PubMed] [Google Scholar]
- 56.Fuller GN, Jacobs JM, Guiloff RJ. Nature and incidence of peripheral nerve syndromes in HIV infection. J Neurol Neurosurg Psychiatry. 1993;56:372–381. doi: 10.1136/jnnp.56.4.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gijtenbeek JM, van den Bent MJ, Vecht CJ. Cyclosporine neurotoxicity: A review. J Neurol. 1999;246:339–346. doi: 10.1007/s004150050360. [DOI] [PubMed] [Google Scholar]
- 58.Gildenberg PL, Game JC, Jr, Kim JH. Stereotactic biopsy of cerebral lesions in AIDS. Clin Infect Dis. 2000;30:491–499. doi: 10.1086/313685. [DOI] [PubMed] [Google Scholar]
- 59.Gozlan J. Cytomegalovirus DNA or RNA detection in blood: Importance of the specimens chosen for amplification (letter) J Infect Dis. 1995;172:1641. doi: 10.1093/infdis/172.6.1641. [DOI] [PubMed] [Google Scholar]
- 60.Gozlan J, el Amrani M, Baudrimont M, et al. A prospective evaluation of clinical criteria and polymerase chain reaction assay of cerebrospinal fluid for the diagnosis of cytomegalovirus-related neurological diseases during AIDS. Aids. 1995;9:253–260. [PubMed] [Google Scholar]
- 61.Graus F, Saiz A, Sierra J, et al. Neurologic complications of autologous and allogeneic bone marrow transplantation in patients with leukemia: A comparative study. Neurology. 1996;46:1004–1009. doi: 10.1212/wnl.46.4.1004. [DOI] [PubMed] [Google Scholar]
- 62.Griffiths PD, Clark DA, Emery VC. Betaherpesviruses in transplant recipients. J Antimicrob Chemother. 2000;45(Suppl T3):29–34. doi: 10.1093/jac/45.suppl_4.29. [DOI] [PubMed] [Google Scholar]
- 63.Grose C, Henle W, Henle G, Feorino PM. Primary Epstein-Barr-virus infections in acute neurologic diseases. N Engl J Med. 1975;292:392–395. doi: 10.1056/NEJM197502202920804. [DOI] [PubMed] [Google Scholar]
- 64.Gross ML, Sweny P, Pearson RM, et al. Rejection encephalopathy. An acute neurological syndrome complicating renal transplantation. J Neurol Sci. 1982;56:23–34. doi: 10.1016/0022-510x(82)90058-2. [DOI] [PubMed] [Google Scholar]
- 65.Hagensee ME, Bauwens JE, Kjos B, Bowden RA. Brain abscess following marrow transplantation: Experience at the Fred Hutchinson Cancer Research Center, 1984–1992. Clin Infect Dis. 1994;19:402–408. doi: 10.1093/clinids/19.3.402. [DOI] [PubMed] [Google Scholar]
- 66.Hall CD, Dafni U, Simpson D, et al. Failure of cytarabine in progressive multifocal leukoencephalopathy associated with human immunodeficiency virus infection. AIDS Clinical Trials Group 243 Team. N Engl J Med. 1998;338:1345–1351. doi: 10.1056/NEJM199805073381903. [DOI] [PubMed] [Google Scholar]
- 67.Hamilton-Dutoit SJ, Pallesen G, Franzmann MB, et al. AIDS-related lymphoma. Histopathology, immunophenotype, and association with Epstein-Barr virus as demonstrated by in situ nucleic acid hybridization. Am J Pathol. 1991;138:149–163. [PMC free article] [PubMed] [Google Scholar]
- 68.Han CS, Miller W, Haake R, Weisdorf D. Varicella zoster infection after bone marrow transplantation: Incidence, risk factors and complications. Bone Marrow Transplant. 1994;13:277–283. [PubMed] [Google Scholar]
- 69.Holland NR, Power C, Mathews VP, et al. Cytomegalovirus encephalitis in acquired immunodeficiency syndrome (AIDS) Neurology. 1994;44:507–514. doi: 10.1212/wnl.44.3_part_1.507. [DOI] [PubMed] [Google Scholar]
- 70.Holloway RG, Mushlin Al. Intracranial mass lesions in acquired immunodeficiency syndrome: Using decision analysis to determine the effectiveness of stereotactic brain biopsy. Neurology. 1996;46:1010–1015. doi: 10.1212/wnl.46.4.1010. [DOI] [PubMed] [Google Scholar]
- 71.Hooper DC, Pruitt AA, Rubin RH. Central nervous system infection in the chronically immunosuppressed. Medicine (Baltimore) 1982;61:166–188. doi: 10.1097/00005792-198205000-00004. [DOI] [PubMed] [Google Scholar]
- 72.Hornef MW, Iten A, Maeder P, et al. Brain biopsy in patients with acquired immunodeficiency syndrome: Diagnostic value, clinical performance, and survival time. Arch Intern Med. 1999;159:2590–2596. doi: 10.1001/archinte.159.21.2590. [DOI] [PubMed] [Google Scholar]
- 73.Hughes BA, Kimmel DW, Aksamit AJ. Herpes zoster-associated meningoencephalitis in patients with systemic cancer. Mayo Clin Proc. 1993;68:652–655. doi: 10.1016/s0025-6196(12)60600-4. [DOI] [PubMed] [Google Scholar]
- 74.Jabbour N, Reyes J, Kusne S, et al. Cryptococcal meningitis after liver transplantation. Transplantation. 1996;61:146–149. doi: 10.1097/00007890-199601150-00027. [DOI] [PubMed] [Google Scholar]
- 75.Kahan BD, Flechner SM, Lorber MI, et al. Complications of cyclosporine-prednisone immunosuppression in 402 renal allograft recipients exclusively followed at a single center for from one to five years. Transplantation. 1987;43:197–204. doi: 10.1097/00007890-198702000-00007. [DOI] [PubMed] [Google Scholar]
- 76.Ketchel SJ, Rodriguez V. Acute infections in cancer patients. Semin Oncol. 1978;5:167–179. [PubMed] [Google Scholar]
- 77.Kim YS, Hollander H. Polyradiculopathy due to cytomegalovirus: Report of two cases in which improvement occurred after prolonged therapy and review of the literature. Clin Infect Dis. 1993;17:32–37. doi: 10.1093/clinids/17.1.32. [DOI] [PubMed] [Google Scholar]
- 78.Knox KK, Carrigan DR. Active human herpesvirus (HHV-6) infection of the central nervous system in patients with AIDS. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;9:69–73. [PubMed] [Google Scholar]
- 79.Kondo K, Nagafuji H, Hata A, et al. Association of human herpesvirus 6 infection of the central nervous system with recurrence of febrile convulsions. J Infect Dis. 1993;167:1197–1200. doi: 10.1093/infdis/167.5.1197. [DOI] [PubMed] [Google Scholar]
- 80.Kondziolka D, Lunsford LD. Results and expectations with image-integrated brainstem stereotactic biopsy. Surg Neurol. 1995;43:558–562. doi: 10.1016/0090-3019(95)00009-7. [DOI] [PubMed] [Google Scholar]
- 81.Koralnik IJ, Boden D, Mai VX, et al. JC virus DNA load in patients with and without progressive multifocal leukoencephalopathy. Neurology. 1999;52:253–260. doi: 10.1212/wnl.52.2.253. [DOI] [PubMed] [Google Scholar]
- 82.Kox LF, Kuijper S, Kolk AH. Early diagnosis of tuberculous meningitis by polymerase chain reaction. Neurology. 1995;45:2228–2232. doi: 10.1212/wnl.45.12.2228. [DOI] [PubMed] [Google Scholar]
- 83.Lakeman FD, Whitley RJ. Diagnosis of herpes simplex encephalitis: Application of polymerase chain reaction to cerebrospinal fluid from brain-biopsied patients and correlation with disease. National Institute of Allergy and Infectious Disease Collaborative Antiviral Study Group. J Infect Dis. 1995;171:857–863. doi: 10.1093/infdis/171.4.857. [DOI] [PubMed] [Google Scholar]
- 84.Larsen RA, Leal MA, Chan LS. Fluconazole compared with amphotericin B plus flucytosine for cryptococcal meningitis in AIDS. A randomized trial. Ann Intern Med. 1990;113:183–187. doi: 10.7326/0003-4819-113-3-183. [DOI] [PubMed] [Google Scholar]
- 85.Leiguarda R, Berthier M, Starkstein S, et al. Ischemic infarction in 25 children with tuberculous meningitis. Stroke. 1988;19:200–204. doi: 10.1161/01.str.19.2.200. [DOI] [PubMed] [Google Scholar]
- 86.Levine SM, Angel L, Anzueto A, et al. A low incidence of posttransplant lymphoproliferative disorder in 109 lung transplant recipients. Chest. 1999;116:1273–1277. doi: 10.1378/chest.116.5.1273. [DOI] [PubMed] [Google Scholar]
- 87.Levy RM, Bredesen DE, Rosenblum ML. Neurological manifestations of the acquired immunodeficiency syndrome (AIDS): Experience at UCSF and review of the literature. J Neurosurg. 1985;62:475–495. doi: 10.3171/jns.1985.62.4.0475. [DOI] [PubMed] [Google Scholar]
- 88.Limaye AP, Huang ML, Atienza EE, et al. Detection of Epstein-Barr virus DNA in sera from transplant recipients with lymphoproliferative disorders. J Clin Microbiol. 1999;37:1113–1116. doi: 10.1128/jcm.37.4.1113-1116.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lin JJ, Harn HJ, Hsu YD, et al. Rapid diagnosis of tuberculous meningitis by polymerase chain reaction assay of cerebrospinal fluid. J Neurol. 1995;242:147–152. doi: 10.1007/BF00936887. [DOI] [PubMed] [Google Scholar]
- 90.Lipton SA. Memantine prevents HIV coat protein-induced neuronal injury in vitro. Neurology. 1992;42:1403–1405. doi: 10.1212/wnl.42.7.1403. [DOI] [PubMed] [Google Scholar]
- 91.Lortholary O, Rozenberg F, Perronne C, et al. Herpes simplex virus type 2 DNA in cerebrospinal fluid of a woman with recurrent meningitis (letter) Clin Infect Dis. 1993;17:941–942. doi: 10.1093/clinids/17.5.941. [DOI] [PubMed] [Google Scholar]
- 92.Luft BJ, Naot Y, Araujo FG, et al. Primary and reactivated toxoplasma infection in patients with cardiac transplants. Clinical spectrum and problems in diagnosis in a defined population. Ann Intern Med. 1983;99:27–31. doi: 10.7326/0003-4819-99-1-27. [DOI] [PubMed] [Google Scholar]
- 93.Luft BJ, Remington JS. AIDS commentary. Toxoplasmic encephalitis. J Infect Dis. 1988;157:1–6. doi: 10.1093/infdis/157.1.1. [DOI] [PubMed] [Google Scholar]
- 94.Lunsford LD, Martinez AJ. Stereotactic exploration of the brain in the era of computed tomography. Surg Neurol. 1984;22:222–230. doi: 10.1016/0090-3019(84)90003-x. [DOI] [PubMed] [Google Scholar]
- 95.Lusso P, Gallo RC. Human herpesvirus 6 in AIDS. Immunol Today. 1995;16:67–71. doi: 10.1016/0167-5699(95)80090-5. [DOI] [PubMed] [Google Scholar]
- 96.Mackenzie IR, Carrigan DR, Wiley CA. Chronic myelopathy associated with human herpesvirus-6. Neurology. 1995;45:2015–2017. doi: 10.1212/wnl.45.11.2015. [DOI] [PubMed] [Google Scholar]
- 97.MacMahon EM, Glass JD, Hayward SD, et al. Epstein-Barr virus in AIDS-related primary central nervous system lymphoma. Lancet. 1991;338:969–973. doi: 10.1016/0140-6736(91)91837-k. [DOI] [PubMed] [Google Scholar]
- 98.Maschke M, Kastrup O, Esser S, et al. Incidence and prevalence of neurological disorders associated with HIV since the introduction of highly active antiretroviral therapy (HAART) J Neurol Neurosurg Psychiatry. 2000;69:376–380. doi: 10.1136/jnnp.69.3.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.McArthur JC, Nance-Sproson TE, Griffin DE, et al. The diagnostic utility of elevation in cerebrospinal fluid beta 2-microglobulin in HIV-1 dementia. Multicenter AIDS Cohort Study. Neurology. 1992;42:1707–1712. doi: 10.1212/wnl.42.9.1707. [DOI] [PubMed] [Google Scholar]
- 100.McCullers JA, Lakeman FD, Whitley RJ. Human herpesvirus 6 is associated with focal encephalitis. Clin Infect Dis. 1995;21:571–576. doi: 10.1093/clinids/21.3.571. [DOI] [PubMed] [Google Scholar]
- 101.McCutchan JA. Cytomegalovirus infections of the nervous system in patients with AIDS. Clin Infect Dis. 1995;20:747–754. doi: 10.1093/clinids/20.4.747. [DOI] [PubMed] [Google Scholar]
- 102.McGuire D, Barhite S, Hollander H, Miles M. JC virus DNA in cerebrospinal fluid of human immunodeficiency virus-infected patients: Predictive value for progressive multifocal leukoencephalopathy (published erratum appears in Ann Neurol 37:687, 1995) Ann Neurol. 1995;37:395–399. doi: 10.1002/ana.410370316. [DOI] [PubMed] [Google Scholar]
- 103.Mehta J, Powles R, Singhal S, et al. Antimicrobial prophylaxis to prevent opportunistic infections in patients with chronic lymphocytic leukemia after allogeneic blood or marrow transplantation. Leuk Lymphoma. 1997;26:83–88. doi: 10.3109/10428199709109161. [DOI] [PubMed] [Google Scholar]
- 104.Meyers BR, Halpern M, Sheiner P, et al. Tuberculosis in liver transplant patients. Transplantation. 1994;58:301–316. [PubMed] [Google Scholar]
- 105.Miaux Y, Ribaud P, Williams M, et al. MR of cerebral aspergillosis in patients who have had bone marrow transplantation. AJNR Am J Neuroradiol. 1995;16:555–562. [PMC free article] [PubMed] [Google Scholar]
- 106.Michaels MG, Wald ER, Fricker FJ, et al. Toxoplasmosis in pediatric recipients of heart transplants. Clin Infect Dis. 1992;14:847–851. doi: 10.1093/clinids/14.4.847. [DOI] [PubMed] [Google Scholar]
- 107.Michaels SH, Clark R, Kissinger P. Incidence and spectrum of AIDS-defining illnesses among persons treated with antiretroviral drugs (letter) Clin Infect Dis. 1999;29:468–469. doi: 10.1086/520251. [DOI] [PubMed] [Google Scholar]
- 108.Olsen WL, Longo FM, Mills CM, Norman D. White matter disease in AIDS: Findings at MR imaging. Radiology. 1988;169:445–448. doi: 10.1148/radiology.169.2.3174991. [DOI] [PubMed] [Google Scholar]
- 109.Patchell RA. Neurological complications of organ transplantation. Ann Neurol. 1994;36:688–703. doi: 10.1002/ana.410360503. [DOI] [PubMed] [Google Scholar]
- 110.Picard FJ, Dekaban GA, Silva J, Rice GP. Mollaref’s meningitis associated with herpes simplex type 2 infection. Neurology. 1993;43:1722–1727. doi: 10.1212/wnl.43.9.1722. [DOI] [PubMed] [Google Scholar]
- 111.Porter SB, Sande MA. Toxoplasmosis of the central nervous system in the acquired immunodeficiency syndrome. N Engl J Med. 1992;327:1643–1648. doi: 10.1056/NEJM199212033272306. [DOI] [PubMed] [Google Scholar]
- 112.Powderly WG. Therapy for cryptococcal meningitis in patients with AIDS. Clin Infect Dis. 1992;14 (Suppl 1):S54–S59. doi: 10.1093/clinids/14.supplement_1.s54. [DOI] [PubMed] [Google Scholar]
- 113.Powderly WG, Cloud GA, Dismukes WE, Saag MS. Measurement of cryptococcal antigen in serum and cerebrospinal fluid: Value in the management of AIDS-associated cryptococcal meningitis. Clin Infect Dis. 1994;18:789–792. doi: 10.1093/clinids/18.5.789. [DOI] [PubMed] [Google Scholar]
- 114.Price RW. Neurological complications of HIV infection. Lancet. 1996;348:445–452. doi: 10.1016/S0140-6736(95)11035-6. [DOI] [PubMed] [Google Scholar]
- 115.Price RW, Yiannoutsos CT, Clifford DB, et al. Neurological outcomes in late HIV infection: Adverse impact of neurological impairment on survival and protective effect of antiviral therapy. AIDS Clinical Trial Group and Neurological AIDS Research Consortium study team. Aids. 1999;13:1677–1685. doi: 10.1097/00002030-199909100-00011. [DOI] [PubMed] [Google Scholar]
- 116.Puchhammer-Stockl E, Popow-Kraupp T, Heinz FX, et al. Detection of varicella-zoster virus DNA by polymerase chain reaction in the cerebrospinal fluid of patients suffering from neurological complications associated with chicken pox or herpes zoster. J Clin Microbiol. 1991;29:1513–1516. doi: 10.1128/jcm.29.7.1513-1516.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Qunibi WY, al-Sibai MB, Taher S, et al. Mycobacterial infection after renal transplantation-report of 14 cases and review of the literature. QJM. 1990;77:1039–1060. doi: 10.1093/qjmed/77.1.1039. [DOI] [PubMed] [Google Scholar]
- 118.Reed MD, Blumer JL. Clinical pharmacology of antitubercular drugs. Pediatr Clin North Am. 1983;30:177–193. doi: 10.1016/s0031-3955(16)34328-0. [DOI] [PubMed] [Google Scholar]
- 119.Rowley AH, Whitley RJ, Lakeman FD, Wolinsky SM. Rapid detection of herpes simplex-virus DNA in cerebrospinal fluid of patients with herpes simplex encephalitis. Lancet. 1990;335:440–441. doi: 10.1016/0140-6736(90)90667-t. [DOI] [PubMed] [Google Scholar]
- 120.Rusthoven JJ, Ahlgren P, Elhakim T, et al. Varicella-zoster infection in adult cancer patients. A population study. Arch Intern Med. 1988;148:1561–1566. [PubMed] [Google Scholar]
- 121.Saag MS, Powderly WG, Cloud GA, et al. Comparison of amphotericin B with fluconasole in the treatment of acute AIDS-associated cryptococcal meningitis. The NIAID Mycoses Study Group and the AIDS Clinical Trials Group. N Engl J Med. 1992;326:83–89. doi: 10.1056/NEJM199201093260202. [DOI] [PubMed] [Google Scholar]
- 122.Sanchez-Portocarrero J, Perez-Cecilia E, Jimenez-Escrig A, et al. Tuberculous meningitis. Clinical characteristics and comparison with cryptococcal meningitis in patients with human immunodeficiency virus infection. Arch Neurol. 1996;53:671–676. doi: 10.1001/archneur.1996.00550070109018. [DOI] [PubMed] [Google Scholar]
- 123.Scarpellini P, Racca S, Cinque P, et al. Nested polymerase chain reaction for diagnosis and monitoring treatment response in AIDS patients with tuberculous meningitis. Aids. 1995;9:895–900. doi: 10.1097/00002030-199508000-00010. [DOI] [PubMed] [Google Scholar]
- 124.Singh N, Carrigan DR, Gayowski T, Marino IR. Human herpesvirus-6 infection in liver transplant recipients: Documentation of pathogenicity. Transplantation. 1997;64:674–678. doi: 10.1097/00007890-199709150-00002. [DOI] [PubMed] [Google Scholar]
- 125.Small SL, Fukui MB, Bramblett GT, Eidelman BH. Immunosuppression-induced leukoencephalopathy from tacrolimus (FK506) Ann Neurol. 1996;40:575–580. doi: 10.1002/ana.410400406. [DOI] [PubMed] [Google Scholar]
- 126.Smirniotopoulos JG, Koeller KK, Nelson AM, Murphy FM. Neuroimaging-autopsy correlations in AIDS. Neuroimaging Clin North Am. 1997;7:615–637. [PubMed] [Google Scholar]
- 127.So YT, Olney RK. Acute lumbosacral polyradiculopathy in acquired immunodeficiency syndrome: Experience in 23 patients. Ann Neurol. 1994;35:53–58. doi: 10.1002/ana.410350109. [DOI] [PubMed] [Google Scholar]
- 128.Taouflk Y, Gasnault J, Karaterki A, et al. Prognostic value of JC virus load in cerebrospinal fluid of patients with progressive multifocal leukoencephalopathy. J Infect Dis. 1998;178:1816–1820. doi: 10.1086/314496. [DOI] [PubMed] [Google Scholar]
- 129.Tenant-Flowers M, Boyle MJ, Carey D, et al. Sulphadiazine desensitization in patients with AIDS and cerebral toxoplasmosis. Aids. 1991;5:311–315. doi: 10.1097/00002030-199103000-00011. [DOI] [PubMed] [Google Scholar]
- 130.Veenstra J, van Praag RM, Krol A, et al. Complications of varicella zoster virus reactivation in HIV-infected homosexual men. Aids. 1996;10:393–399. doi: 10.1097/00002030-199604000-00007. [DOI] [PubMed] [Google Scholar]
- 131.Verhagen WI, Huygen PL, Dalman JE. Central nervous system Whipple’s disease (letter) Ann Neurol. 1997;41:560–561. doi: 10.1002/ana.410410424. [DOI] [PubMed] [Google Scholar]
- 132.Visser LH, van der Meche FG, Meulstee J, et al. Cytomegalovirus infection and Guillain-Barré syndrome: The clinical, electrophysiologic, and prognostic features. Dutch Guillain-Barré Study Group. Neurology. 1996;47:668–673. doi: 10.1212/wnl.47.3.668. [DOI] [PubMed] [Google Scholar]
- 133.Voges J, Schroder R, Treuer H, et al. CT-guided and computer assisted stereotactic biopsy. Technique, results, indications. Acta Neurochir. 1993;125:142–149. doi: 10.1007/BF01401842. [DOI] [PubMed] [Google Scholar]
- 134.Walot I, Miller BL, Chang L, Mehringer CM. Neuroimaging findings in patients with AIDS. Clin Infect Dis. 1996;22:906–919. doi: 10.1093/clinids/22.6.906. [DOI] [PubMed] [Google Scholar]
- 135.Whitley RJ. Neonatal herpes simplex virus infections. J Med Virol. 1993;(Suppl):13–21. doi: 10.1002/jmv.1890410505. [DOI] [PubMed] [Google Scholar]
- 136.Whitley RJ, Gnann JW., Jr Acyclovir: A decade later. N Engl J Med. 1992;327:782–789. doi: 10.1056/NEJM199209103271108. [DOI] [PubMed] [Google Scholar]
- 137.Whitley RJ, Soong SJ, Hirsch MS, et al. Herpes simplex encephalitis: Vidarabine therapy and diagnostic problems. N Engl J Med. 1981;304:313–318. doi: 10.1056/NEJM198102053040602. [DOI] [PubMed] [Google Scholar]
- 138.Winnock S, Janvier G, Parmentier F, et al. Pontine myelinolysis following liver transplantation: A report of two cases. Transpl Int. 1993;6:26–28. doi: 10.1007/BF00336635. [DOI] [PubMed] [Google Scholar]
- 139.Yamanishi K, Okuno T, Shiraki K, et al. Identification of human herpesvirus-6 as a causal agent for exanthem subitum. Lancet. 1998;1:1065–1067. doi: 10.1016/s0140-6736(88)91893-4. [DOI] [PubMed] [Google Scholar]
- 140.Yechoor VK, Shandera WX, Rodriguez P, Gate TR. Tuberculous meningitis among adults with and without HIV infection. Experience in an urban public hospital (published erratum appears in Arch Intern Med 12;157:1022, 1997) Arch Intern Med. 1996;156:1710–1716. [PubMed] [Google Scholar]