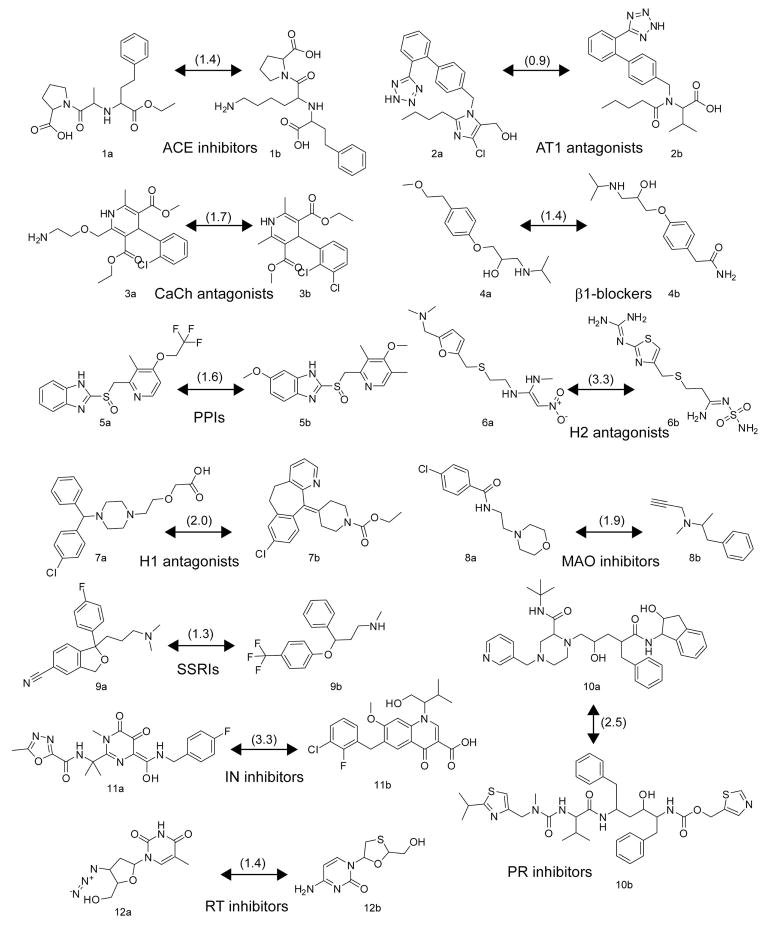
Figure 4.
Chemical structures of drugs with shared mode of action used to calculate within group EDs; the angiotensin-converting enzyme (ACE) inhibitors enalapril (1a) and lisinopril (1b), the angiotensin receptor 1 (AT1) antagonists losartan (2a) and valsartan (2b), the calcium channel (CaCh) blockers amlodipine (3a) and felodipine (3b), the β1–adrenergic receptor (β1) blockers metoprolol (4a) and atenolol (4b), the proton pump inhibitors (PPIs) lansoprazole (5a) and omeprazole (5b), the histamine 2 (H2) receptor antagonists ranitidine (6a) and famotidine (6b), the H1 receptor antagonists cetirizine (7a) and loratidine (7b), the monoamine oxidase (MAO) inhibitors moclobemide (8a) and selegeline (8b), the selective serotonin re-uptake inhibitors (SSRIs) citalopram (9a) and fluoxetine (9b), the HIV-1 protease (PR) inhibitors indinavir (10a) and ritonavir (10b), the HIV-1 integrase (IN) inhibitors raltegravir (11a) and elvitegravir (11b), and the HIV-1 reverse transcriptase (RT) enzyme inhibitors zidovudine (12a), and lamivudine (12b). EDs are given in parentheses.