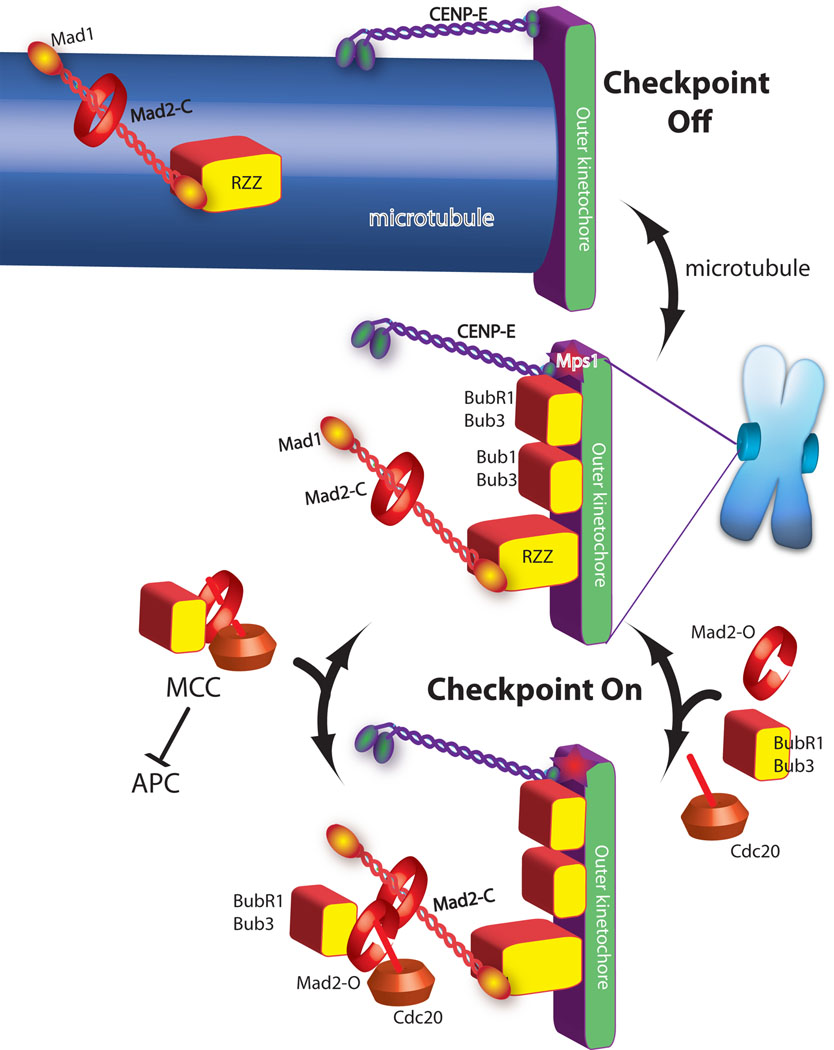
Figure 1. A current model of spindle checkpoint signaling highlights the importance of MCC formation as the step catalyzed by the kinetochore.
Green and purple are proteins required for microtubule binding. Checkpoint proteins (red and yellow shapes) are recruited to kinetochores that are not binding microtubules (central image). In the absence of microtubules the checkpoint proteins act to catalyze the assembly of the MCC checkpoint complex (bottom image), which diffuses from the kinetochore to inhibit the anaphase promoting complex (APC). Mad2 has both open and closed states and although it is a globular protein it is drawn as a ring to emphasize transitions between these two states. Mad2closed on kinetochores binds Mad2open, which in turn binds Cdc20, Bub1 and Bub3 to form MCC.
Microtubule attachment inhibits the signal by two mechanisms. The checkpoint proteins Mad1/Mad2 and RZZ are shown being “stripped” by dynein, which carries them away from the kinetochore by walking towards the minus end of the microtubule (top image). CENP-E activates BubR1 kinase activity unless it binds microtubules, which is also important for silencing the checkpoint.