Figure 3. Structural model of the GABAA receptor second transmembrane domain.
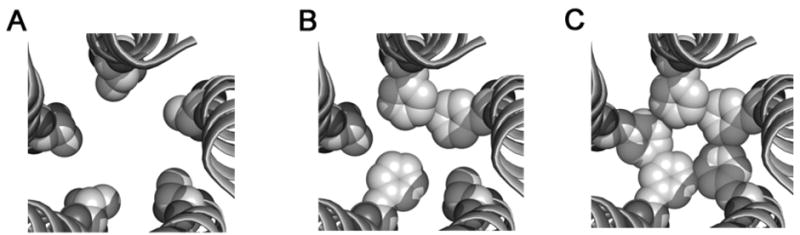
Proposed model of the TM2 domains in the open state containing five threonines (A), three (B) and five phenylalanines (C) at the 6′ position. The residue at this position (space-filled representation) is channel-lining [17]. The pore is larger with the five threonines (A) and constricts as more 6′ residues are converted to phenylalanines (C), as what may be observed for α1(T6′F)β2(T6′F) or α1(T6′F) β2 (T6′F)γ2(T6′F) mutant receptors. The inclusion of 2 or more phenylalanines could interact hydrophobically to move the receptor from the open state to a desensitized state.
