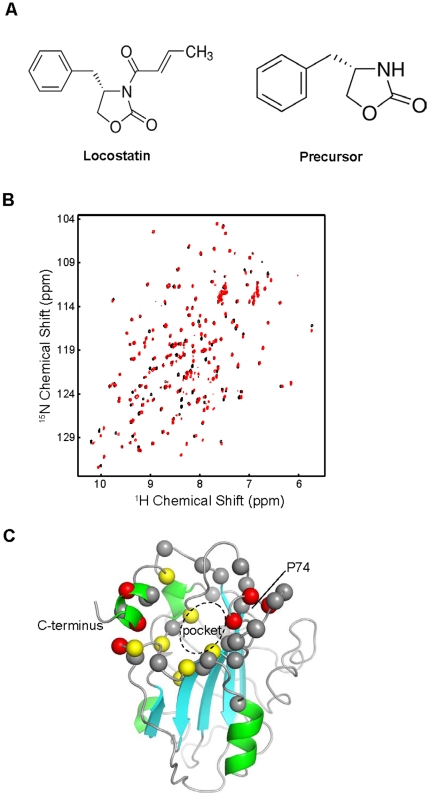
Figure 1. Binding of the locostatin precursor (S)-4-benzyl-2-oxazolidinone (Sigma #294640) to RKIP characterized by NMR chemical shift perturbation.
(A) Chemical structure of locostatin and its precursor (S)-4-benzyl-2-oxazolidinone. (B) Comparison of 1H,15N-HSQC spectra of 15N-enriched RKIP in the absence-black and the presence-red of the compound (1 mM). Each cross peak (dot) represents the correlation between the directly bonded amide 1H and 15N atoms of amino acid residue. (C) The locations of amino acid residues whose HSQC peak is significantly affected by compound binding mapped on the RKIP structure. Red, yellow and gray spheres represent residues that have the weighted shift of >0.3 ppm, 0.2> ppm and >0.1 ppm, respectively.