Abstract
Obesity is a leading cause of diabetes mellitus and hypertension. Molecular signals produced by adipose tissue may contribute to the pathogenesis of these two disorders. We showed previously that a specific segment of rat chromosome 20 (RNO20) contains a gene(s) regulating the degree of obesity, glucose intolerance, and hypertension in response to a chronic high-fat diet (HFD). Here we examined microarray gene expression profiles and cellular morphology of adipose tissues and whole body energy expenditure in this model. Adult male spontaneously hypertensive rats (SHR) and a congenic strain (SHR.1N) that differs from SHR by the above-mentioned segment of RNO20 were fed for 12 wk with HFD or a normal diet. At the end of this period, whole body energy expenditure was measured with indirect calorimetry. In response to HFD, body weight, fat pad weights, adipocyte size, and serum leptin levels increased significantly more in SHR.1N than SHR. Microarray gene expression profiles [Affymetrix, 15,923 genes and expressed sequence tags (ESTs)] showed that multiple genes of molecular pathways involved in lipogenesis were downregulated to a similar level in both strains, whereas genes involved in fatty acid oxidation and energy dissipation were upregulated less in SHR.1N than SHR. This was associated with lower whole body energy expenditure in SHR.1N than SHR at the end of the 12-wk HFD. Our results suggest that a gene(s) within the RNO20 segment regulate(s) HFD-induced increases in adiposity, and that this effect may be mediated, at least in part, by the impact of that gene(s) on fat burning and energy expenditure.
Keywords: obesity, genetics, microarray gene expression profiling
the environment created by modern industrialized societies, characterized by increased energy intake and decreased physical activity, resulted in an unprecedented rise in the prevalence of obesity and obesity-related disorders, including diabetes mellitus and hypertension (8, 24). The mechanisms underlying the link between obesity and these disorders are thought to be related to an excess of “dysfunctional” adipose tissue producing molecular signals that, for example, impair insulin sensitivity, promote low-grade systemic inflammation, or increase sympathetic activity and the production of reactive oxygen species (9, 27, 42).
Obesity, a condition defined by an excess of adipose tissue, develops as a consequence of energy intake exceeding energy expenditure. The growth of adipose tissue results from hypertrophy of existing adipocytes and/or from the generation of new adipocytes (hyperplasia). Adipocyte hypertrophy is mainly due to the accumulation of intracellular triacylglycerols, whereas hyperplastic growth is attributed predominantly to the differentiation of resident preadipocytes and mesenchymal progenitor cells (34). Both hypertrophy and hyperplasia involve complex cascades of transcriptional and nontranscriptional events that begin in the prenatal period and continue throughout life (33). Mesenchymal progenitor cells proliferate and differentiate into preadipocytes (cells macroscopically indistinguishable from fibroblasts) that, under appropriate nutritional and hormonal conditions, differentiate into mature adipocytes. During this last step, the cells become larger and rounder, and their cytoplasm fills with multiple lipid droplets, which ultimately fuse into a single large droplet. Alongside these morphological changes, adipocytes alter the production of molecular signals that may be involved in the development of obesity-related disorders, such as diabetes mellitus and hypertension (1, 34).
The causal role of environment in the current epidemic of obesity is undeniable. Genetic makeup, however, plays an important role in that it modifies individuals' susceptibility to both obesity itself and obesity-related disorders (30). Consistent with this view, we (28) and others (7, 16, 25, 36, 37) have demonstrated that the human tumor necrosis factor (TNF)-α gene locus on chromosome 6 is an important determinant of obesity and obesity-associated hypertension and insulin resistance. The TNF-α gene was chosen as a candidate gene in these studies because it encodes a pluripotent cytokine that is produced by adipose tissue (42) and that is known to be involved in the regulation of adipogenesis, lipogenesis, and systemic insulin sensitivity (6, 17, 26). In addition to human investigations, we carried out a rat study (29) in which we showed that a region of rat chromosome 20 (RNO20) that is homologous to the human TNF-α gene locus determines increases in adiposity, glucose intolerance, and blood pressure in response to a chronic high-fat diet (HFD) and that the effect of the rat Tnfα gene region on adiposity is not mediated by its influence on food intake or locomotor activity. Given the potential importance of adipose tissue in the development of obesity-related glucose intolerance and hypertension, the aim of the present study was to further our findings of the rat study and perform histological analyses and microarray gene expression profiling in adipose tissue.
MATERIALS AND METHODS
Animals
Spontaneously hypertensive rats (SHR/Ola) and a congenic strain, SHR.BN-D20Cebr215s7-D20Rat23 (hereafter referred to as SHR.1N), derived from SHR/Ola and normotensive Brown Norway (BN.Lx) rats, were studied. The congenic strain SHR.1N differs from SHR by a segment of RNO20 that has been transferred onto the SHR background from BN.Lx. The differential chromosomal segment of SHR.1N is ∼21 Mb long and contains the Tnfα gene (32).
Experimental Protocol
Sixteen-week-old male SHR and SHR.1N rats were placed on a normal diet (ND, 5P14, Prolab 2500; 3.4 kcal/g; 20% protein, 15% fat, and 65% carbohydrates) or HFD (F3282, Bio-Serv; 5.3 kcal/g; 15% protein, 60% fat, and 25% carbohydrates) for 12 wk (SHR/ND: n = 11, SHR/HFD: n = 15, SHR.1N/ND: n = 9, and SHR.1N/HFD: n = 15). During the 12-wk period, body weight and food consumption were monitored weekly. At the end of the intervention, the animals were humanely killed after regular overnight feeding between 0800 and 1200, in an order systematically rotating among the four experimental groups, and sera and adipose tissues were collected. Epididymal and retroperitoneal fat pads were quickly removed, weighed, snap-frozen in liquid nitrogen, and, together with collected sera, stored at −80°C until further analysis.
Whole Body Energy Expenditure
In addition to the above experiment, we carried out an auxiliary study in which we measured metabolic rate together with locomotor activity and food intake in an additional set of male SHR and SHR.1N rats at the end of 12-wk HFD (SHR: n = 4, SHR.1N: n = 3) or ND (SHR: n = 4, SHR.1N: n = 5). Age of animals and diets given were the same as in the above experiment. Oxygen consumption (V̇o2) and carbon dioxide production (V̇co2), as indexes of whole body energy expenditure, as well as locomotor activity and food intake were measured for two consecutive 24-h periods with a Comprehensive Lab Animal Monitoring System (CLAMS; Linton Instrumentation, Linton, UK; Columbus Instruments, Columbus, OH). This is a modified open-circuit calorimeter consisting of eight chambers in which rats are studied individually. The flow rate of air through the chamber was adjusted to 2 l/min and extracted outflow to 0.4 l/min. The ambient air (input air to the chambers) and the expired air were analyzed every 13 min. The effluent gas was drawn through a column filled with Drierite (anhydrous CaSO4) placed before a gas analyzer. All measurements were taken at room temperature of 23°C (12:12-h light-dark cycle; dark phase 1900–0700).
The studies have been approved by the Animal Ethics Committee of Centre de Recherche de l'Université de Montréal and the United Kingdom Home Office (PPL 40/2881).
Adipocyte Size
Sections of retroperitoneal and epididymal fat pads were fixed in 3.7% formaldehyde, embedded in paraffin, sliced at a 5-μm thickness, and stained with hematoxylin-phloxin-safran. Sections were visualized microscopically with an Olympus IX71 microscope at ×100 magnification and photographed with a digital camera. In each animal, a cross-sectional area of at least 80 adipocytes randomly selected on 4 tissue slices was measured with Scion Image software (Beta 4.0.2 version). Adipocytes were examined blind to experimental group.
Gene Expression Microarray Profiling
Experimental design, sampling, hybridization, and data analysis and presentation were performed in compliance with Minimum Information About a Microarray Experiment (MIAME) guidelines. More specifically, total RNA was isolated from retroperitoneal fat pads with TRIzol reagent (Invitrogen Canada, Burlington, ON, Canada). The quality of total RNA was evaluated with the Agilent 2100 BioAnalyzer system (Agilent Technologies, Palo Alto, CA). The RNA samples from individual animals were then pooled within each of the four experimental groups (SHR.1N/ND: n = 6, SHR.1N/HFD: n = 6, SHR/ND: n = 8, and SHR/HFD: n = 8) and subjected to gene expression profiling with the Rat RAE-230a GeneChip Set containing 15,923 genes and expressed sequence tags (ESTs; Affymetrix, Santa Clara, CA). A single array was used for each strain and diet group. The pooled RNA samples were prepared for hybridization according to the manufacturer's instructions. MicroArray Suite (version 5, Affymetrix) was used to scale signal intensities across the gene chips to 150 fluorescence units and to determine expression levels for each gene on the chip. GeneSpring 6.0 was used to normalize data both per “chip” (to the median of all elements on each chip) and per “gene” (to the median of that gene on all 4 chips). Only genes that were “present” (P < 0.05) were included in further analyses.
To identify genes influenced by chronic HFD feeding, we then searched among the “present” genes for those demonstrating a ≥1.8-fold difference between HFD- and ND-fed groups in either strain and showing an expression level ≥2.0 (units of signal intensity) in at least one of the four experimental conditions. The expression level of ≥2.0 (units of signal intensity) was chosen because the assessment of gene expression at lower levels is less reliable. Among genes identified in this way, we then searched for those demonstrating a strain-differential response to HFD that was defined as the ≥1.8-fold difference between HFD- and ND-fed groups 1) being present in one of the two strains only, 2) being greater by ≥1.8 fold in one than in the other strain, or 3) being in an opposing direction in the two strains (e.g., upregulated in SHR and downregulated in SHR.1N). Within each of these groups of similarly diet/strain-regulated genes, we searched for those that were functionally related. Gene function and functional clustering were determined by searching through publicly available databases.
Real-time reverse transcription polymerase-chain reaction (RT-PCR) was employed to verify results of gene expression microarray profiling in individual animal RNA samples. The genes evaluated in this way together with their primers are listed in Supplemental Table S1.1 Real-time RT-PCR was carried out with a SensiMix Plus SYBR Green PCR kit (Quantace, London, UK) and a Rotor Gene 3000 cycler (Corbett Research, Sydney, Australia). Reaction efficiency was determined by serial dilutions of a cDNA reference sample. Relative quantification was carried out with the Pfaffl method (31).
Serum Leptin and Serum and Adipose Tissue Tnfα
Serum leptin concentrations were measured by radioimmunoassay (Linco Research, St. Charles, MO), and serum and adipose tissue Tnfα concentrations were quantified by the ELISA method (BDOptEIA set, BD Biosciences, Mississauga, ON, Canada).
Statistical Analyses
Body weight, weights of fat pads, and adipocyte size were analyzed by two-way ANOVA, with main factors being strain (SHR vs. SHR.1N) and diet (ND vs. HFD). Relationships between adipocyte size and serum leptin concentration were evaluated with the Pearson correlation coefficient. Statistical analyses were carried out with JMP (version 5.1.2, SAS Institute).
RESULTS AND DISCUSSION
Adiposity and Adipocyte Size and Its Relationship with Circulating Leptin
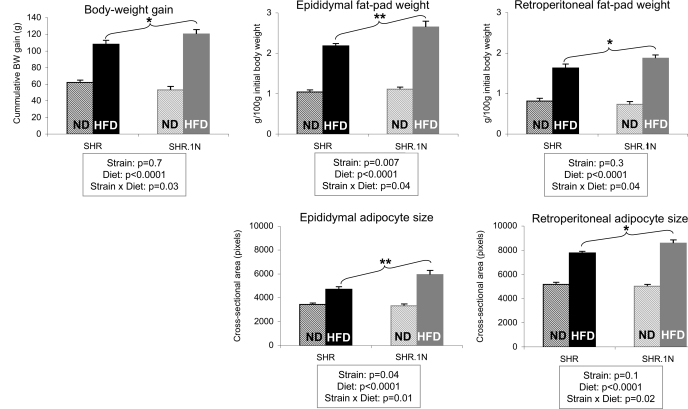
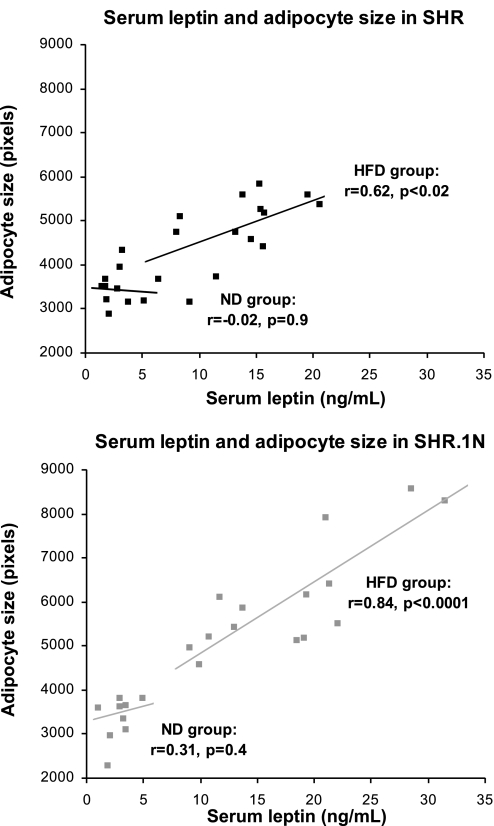
In response to a 12-wk HFD, SHR.1N compared with SHR rats gained significantly more body weight and their epididymal and retroperitoneal fat pads became larger (Fig. 1). These results were similar to those we reported previously (29). In addition, SHR.1N compared with SHR showed a greater epididymal and retroperitoneal adipocyte size (Fig. 1). They also demonstrated higher circulating levels of leptin, as we showed previously (29). It has been suggested that the adipocyte production of leptin is an intrinsic property of adipocyte size (12). Consistent with this proposal, adipocyte size correlated closely with serum leptin levels (Fig. 2), with the correlation being highly significant in SHR.1N fed HFD (explaining 71% of variance) and less significant in SHR rats fed HFD (explaining only 38% of variance). No significant strain differences were seen in food consumption in either ND or HFD groups (SHR.1N/ND: 24.9 ± 1.1, SHR/ND: 23.8 ± 0.9, SHR.1N/HFD: 22.8 ± 1.4, and SHR/HFD: 21.8 ± 1.2 g/day).
Fig. 1.
Adiposity and adipocyte size in spontaneously hypertensive rats (SHR) and SHR.1N. Body weight gain, weight of fat pads, and adipocyte size in SHR and SHR.1N fed for 12 wk with either a high-fat diet (HFD) or a normal diet (ND) are presented. *P < 0.05, **P < 0.01.
Fig. 2.
Serum leptin and adipocyte size in SHR and SHR.1N. Relationship between serum leptin and adipocyte size assessed in retroperitoneal fat pads in SHR and SHR.1N fed for 12 wk with either HFD or ND is presented.
Adipose Tissue Gene Expression Profiles
Results of microarray gene expression profiling in retroperitoneal fat pads demonstrated that, in response to a 12-wk HFD, a total of 153 genes were upregulated or downregulated (≥1.8 times) in SHR or SHR.1N. A number of these genes (n = 36) showed expression profiles that were remarkably similar between the two strains, indicating that their expression is changed by HFD, but the studied segment of RNO20 does not influence these changes. The remaining genes (n = 117) showed various strain-differential responses to HFD, as defined in materials and methods. None of these genes, however, was located within the RNO20 segment. This suggests that expression of these genes is changed by HFD and that a gene(s) within the RNO20 segment modifies them, but the “modifier” gene itself does not show a ≥1.8-fold upregulation or downregulation by HFD or is not present on the Rat RAE-230a GeneChip Set (207 genes of a total of 481 genes residing within the differential RNO20 segment were present on the chip). Alternatively, the “modifier” gene may be functionally altered but not differentially expressed at the mRNA level.
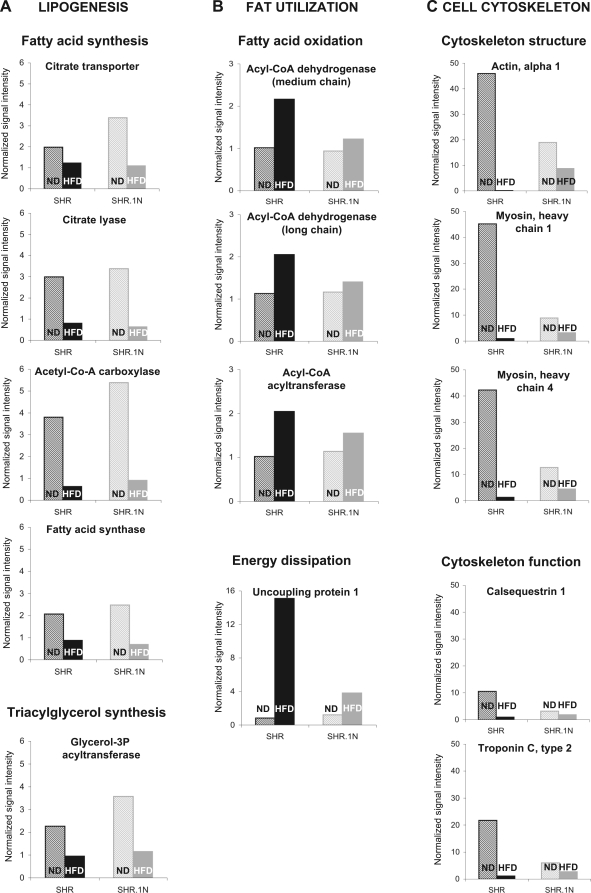
Finally, a relatively large proportion of the HFD-regulated genes (n = 71) were functionally related, and, within each functional cluster, they showed very similar expression profiles. These were genes involved in 1) lipogenesis, 2) fat utilization, and 3) cell cytoskeleton (Fig. 3 and Supplemental Table S2).
Fig. 3.
Microarray gene expression analysis of adipose tissue from SHR and SHR.1N. Expression of representative genes selected from functional clusters, lipogenesis (A), fat utilization (B), and cell cytoskeleton (C), are shown in SHR and SHR.1N fed with either ND or HFD for 12 wk.
Cluster 1: Lipogenesis.
The first cluster included genes (n = 25) encoding enzymes and transport proteins involved in both glycerol and fatty acid (FA) supply for triacylglycerol synthesis and triacylglycerol synthesis itself. These genes showed HFD-induced downregulation in both SHR and SHR.1N (Fig. 3A and Supplemental Table S2A). They included genes of glucose uptake [glucose transporter 4 (Glut4)], glycolysis [hexokinase (Hk2)], intermediary metabolism [pyruvate carboxylase (Pc), pyruvate dehydrogenase complex (Pdh, Pdha1, Pdk1, Dlat), and malic enzyme (Me1)], pentose pathway [transketolase (Tkt)], FA uptake and transport [FA transport protein 1 (Slc27a1) and albumin (Alb)], FA synthesis, elongation, and desaturation [citrate transport protein (Slc25a1), ATP citrate lyase (Acly), cytosolic acetyl-coenzyme-A carboxylase (Acaca), FA synthase (Fas), FA elongase 2 (Elovl6), and steroyl-CoA desaturase 2 (Scd2)], and triacylglycerol synthesis [mitochondrial glycerol-3-phosphate acyltransferase (Gpam)]. More than half of these genes (n = 14) were downregulated similarly in SHR and SHR.1N (Supplemental Table S2A). The remaining genes (n = 11) were downregulated by HFD more in SHR.1N than in SHR. But this difference was mainly due to the expression levels being higher in SHR.1N than SHR in animals fed ND but not in animals fed HFD; there the levels were almost identical (Fig. 3A). Together, these results suggest that, in response to chronic HFD feeding, the capacity of adipose tissue for lipogenesis decreases. Because some of these genes demonstrate higher expression in SHR.1N than SHR fed ND, the relative decrease in expression of these genes was greater in SHR.1N than SHR. Whether this indicates that the capacity for lipogenesis had been higher in SHR.1N vs. SHR before it became downregulated by HFD requires additional short-term experiments.
Cluster 2: Fat utilization.
This cluster included genes (n = 4) involved in FA oxidation and energy dissipation. These genes were upregulated by HFD either in SHR only or more in SHR than in SHR.1N (Fig. 3B and Supplemental Table S2B). The genes of FA oxidation were the medium-chain and long-chain acyl-CoA dehydrogenase genes (Acadm and Acadl) and the mitochondrial acetyl-CoA acyltransferase gene (Acaa2); these genes encode enzymes catalyzing the first and last steps of FA oxidation. They showed HFD-induced upregulation in SHR only (from 1.8- to 2.1-fold; Fig. 3B). The gene of energy dissipation was the uncoupling protein 1 (Ucp1) gene, which demonstrated a 18-fold upregulation in SHR but only a 3-fold upregulation in SHR.1N (Fig. 3B). Ucp1 is a proton transporter localized to the mitochondrial inner membrane, where it can uncouple the oxidation of FAs from ATP production and generate heat instead (13). Typically, Ucp1 is expressed only in brown adipocytes that, in adults, are scattered within white adipose tissue (34). Most prominent expression of Ucp1 can be found in intra-abdominal fat depots, such as the retroperitoneal fat pad studied here (13). Transgenic overexpression of Ucp1 (21) and modest induction of Ucp1 in white adipose tissue (4, 40) results in resistance to HFD-induced obesity; it also improves glucose tolerance (44). These results suggest that chronic HFD feeding may increase the capacity of white adipose tissue to oxidize fat and dissipate energy as heat, and that this effect is greater in SHR than in SHR.1N. As such, it may contribute to the observed development of a smaller adipocyte size and adiposity in SHR than SHR.1N animals fed HFD (Fig. 1).
Cluster 3: Cell cytoskeleton.
The third cluster contained genes of cell cytoskeleton architecture and function. This cluster was the largest one, including 43 genes. All genes within the cluster were downregulated by HFD more in SHR than in SHR.1N (Fig. 3C and Supplemental Table S2D). This cluster of genes included, for example, genes encoding proteins that support cytoskeleton architecture [α-actin (Acta1) and myosin heavy chain (Myh4 and Myh1)] or proteins that support cytoskeleton function [calsequestrin (Casq1) and troponin C2 (Tnnc2)] (Fig. 3C). Our observation of the downregulation of cytoskeleton genes is in agreement with previous studies that demonstrated a downregulation of similar genes (e.g., Acta1) by HFD in mice (20) and a reduction in the cytoskeleton network by up to 90% during adipocyte differentiation (38). Whether these changes are involved in determining the strain differences in adipocyte size and adiposity observed in this study is not clear at present (Fig. 1). The cell cytoskeleton plays an essential role in a variety of cellular processes, including maintenance of cell shape and motility (10, 19); it also determines mechanical properties of cells, such as elasticity and rigidity (10). Furthermore, the fact that all genes in this cluster were of the “skeletal muscle” ontology may indicate that the potential for emergence of brown adipocytes in retroperitoneal adipose tissue may be higher in SHR than SHR.1N, because it has been shown recently that brown adipocytes endogenously share an early transcriptional program with skeletal muscle cells (35, 39).
In addition to the above functional clusters, several other genes that showed HFD-induced changes in expression were related to adipogenesis and lipogenesis. For example, the adipose differentiation-related protein (Adrp) gene demonstrated 4.4- and 5.1-fold upregulation in SHR and SHR.1N, respectively (Supplemental Table S2C). This gene encodes a protein associated with the surface of lipid droplets and may be involved in adipocyte triacylglycerol turnover (23). The matrix metalloproteinase-12 (Mmp12) gene encoding an enzyme involved in extracellular matrix degradation in adipose tissue during its expansion (18) also showed an upregulation in SHR and SHR.1N (2.1- and 3.0-fold, respectively; Supplemental Table S2C). These HFD-induced changes are consistent with previous studies demonstrating that expression of these genes increases in parallel with the development of adipocyte hypertrophy (5, 18, 23).
Adipocyte size correlates positively with the degree of macrophage infiltration of adipose tissue, with macrophages being responsible for almost all adipose tissue expression of Tnfα (42). In the present study, expression of the chemokine-like receptor 1 (Cmklr1) gene, which plays a critical role in macrophage-mediated immune responses (45), was downregulated by HFD and this downregulation was similar in SHR and SHR.1N (3- and 2.1-fold, respectively; Supplemental Table S2E).
The most pronounced strain-differential response to HFD was observed in expression of the aquaporin 3 (Aqp3) gene (upregulated 3.9 times in SHR and downregulated 28.9 times in SHR.1N) (Supplemental Table S2F). Aqp3 functions as a plasma membrane protein that transports glycerol and water (15). Its adipocyte form (Aqp7) was shown recently to play a role in cellular glycerol release (14). Its upregulation resulted in an augmented glycerol exit from adipocytes, decreased glycerol supply for triacylglycerol synthesis, and smaller adipocyte size (14). Thus, if Aqp3 functions in a similar way, the upregulation of Aqp3 observed here could decrease glycerol supply for triglyceride synthesis and, hence, the development of smaller adipocytes in SHR than SHR.1N in animals fed HFD.
The expression profiles obtained with microarrays were verified by real-time RT-PCR in individual animal RNA samples, focusing on the three functional clusters discussed above (Supplemental Table S1). These analyses confirmed that genes involved in lipogenesis (cluster 1) were significantly downregulated by HFD in both strains (Supplemental Fig. S1A). They also confirmed that most genes of fat utilization (cluster 2) were significantly upregulated by HFD in SHR but not in SHR.1N (Supplemental Fig. S1B), and that most genes of cell cytoskeleton (cluster 3) were significantly downregulated by HFD in SHR but not in SHR.1N (Supplemental Fig. S1C).
Apart from verifying the microarray results, the real-time RT-PCR in individual animal RNA samples revealed that certain genes exhibited greater interindividual variability; in some animals they were expressed at a very high level, whereas in others they were expressed either at a very low level or not at all (Supplemental Fig. S2, A and B). These genes included all those in cluster 3 (cell cytoskeleton genes of skeletal muscle ontology) and Ucp1. Interestingly, all of these genes have been implicated in emergence of brown adipocyte islands in white adipose tissue (35, 39). Therefore, we speculate that the observed interindividual variability may relate to the presence or absence of such islands in the sections of fat pads used in our gene expression studies. Moreover, the fact that these genes were regulated by HFD only in SHR and in such a way that the genes of skeletal muscle ontology, shown previously to be transiently expressed at early stages of brown adipogenesis (39), were downregulated by HFD and the gene marker of mature brown adipocyte, Ucp1, was upregulated by HFD suggests that SHR but not SHR.1N may possess a genetic variant within the differential chromosomal segment that promotes brown adipogenesis in response to chronic HFD. Emergence of brown adipocytes within white adipose tissue has been shown previously in response to chronic exposure to cold and by prolonged β-adrenergic stimulation (11). Brown fat can increase energy expenditure through a specialized program of chemical energy dissipation as heat (2).
Whole Body Energy Expenditure
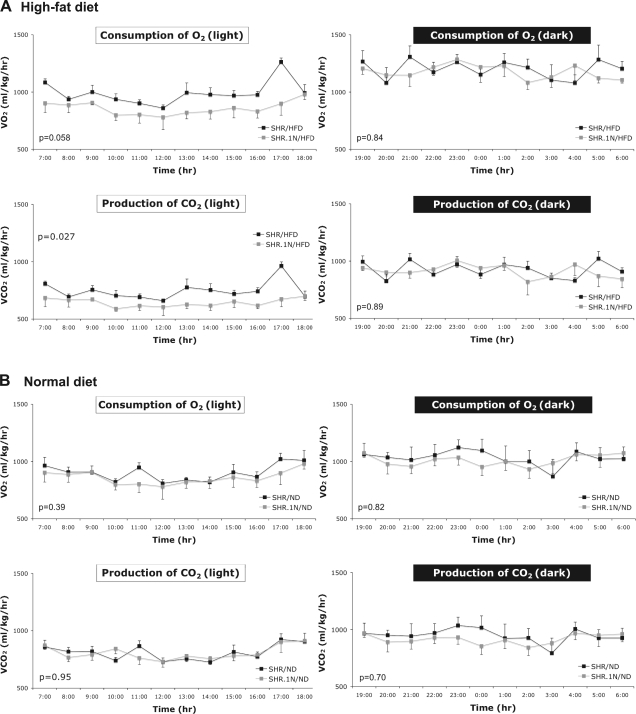
The above results of gene expression analyses suggest that chronic HFD feeding may increase the capacity for energy expenditure and that this effect may be greater in SHR than in SHR.1N. To support these findings, we carried out an auxiliary study in which we measured indexes of metabolic rate, V̇o2 and V̇co2, alongside locomotor activity and food intake for two consecutive 24-h periods at the end of a 12-wk HFD or ND. These analyses revealed that, similar to our main study, SHR.1N compared with SHR tended to gain more body weight in response to HFD (strain × diet interaction: P = 0.09). With respect to whole body energy expenditure, both V̇o2 and V̇co2 were lower in SHR.1N than in SHR when fed HFD, but only during the light (inactive) phase; they did not differ during either phase in animals fed ND (Fig. 4). No significant strain differences were observed in food intake and locomotor activity in either ND-fed (P = 0.2 and P = 0.6, respectively) or HFD-fed (P = 0.5 and P = 0.4, respectively) animals. As such, these results support the findings of the microarray gene expression profiling in that they demonstrate that the capacity for energy expenditure is lower in SHR.1N than in SHR when fed chronically by HFD. The fact that this strain difference was seen only during the light phase is consistent with other studies demonstrating that HFD may induce differences in energy expenditure during this phase between obesity-sensitive and obesity-resistant mice (22).
Fig. 4.
Whole body energy expenditure in SHR and SHR.1N. One-hour averages of oxygen consumption (V̇o2) and carbon dioxide production (V̇co2), as indexes of whole body energy expenditure, were measured during 12-h light and dark phases in SHR and SHR.1N rats fed for 12 wk with either HFD (A) or ND (B).
Serum and Adipose Tissue Tnfα
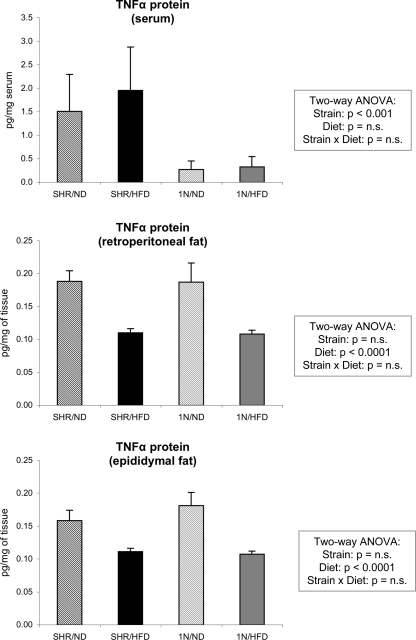
The Tnfα gene, a candidate gene located within the differential chromosomal segment of SHR.1N, inhibits adipogenesis and lipogenesis, and possibly also energy dissipation (3). In our previous study (29), we identified a total of 26 sequence differences in the Tnfα gene between SHR and SHR.1N. None of these differences changes amino acid sequence, but some of them were found in regions that may influence gene and/or protein expression of this cytokine (29). In the same study, we showed that the mRNA expression of the Tnfα gene in retroperitoneal and epididymal adipose tissues is either decreased or unchanged by HFD but, most importantly, does not differ between SHR and SHR.1N (29). Expression of Tnfα is regulated not only at a transcriptional but also at a posttranscriptional level. Therefore, in the present study, we analyzed protein concentrations of Tnfα in both fat pads and the circulation. These analyses showed that in the fat pads Tnfα protein expression is decreased by HFD and that this decrease does not differ between SHR and SHR.1N [similar to our previous mRNA analyses (29)], whereas in the circulation it is not affected by HFD but is significantly higher in SHR than in SHR.1N (Fig. 5). Thus these results provide further evidence against a causal role of the Tnfα gene in the strain-differential effects of HFD on adipocyte size and adiposity. Our finding that the Tnfα protein is downregulated by HFD in both epididymal and retroperitoneal fat pads is not in agreement with some other studies, which demonstrated the opposite (17, 43). Within adipose tissue, almost all Tnfα is expressed by macrophages (42). The decreased Tnfα expression may therefore indicate that macrophage infiltration of adipose tissue does not occur in our model. This possibility is supported by our results of microarray gene expression profiling demonstrating that expression of the Cmklr1 gene, which plays a critical role in regulating macrophage-mediated immune responses (45), was also downregulated by HFD similarly in both strains.
Fig. 5.
Tnfα protein expression in the circulation and retroperitoneal and epididymal fat pads. These data are shown in SHR and SHR.1N (1N) fed with either ND or HFD for 12 wk.
The differential chromosomal segment of SHR.1N is ∼21 Mb long, and, in addition to the Tnfα gene, it contains 481 genes (http://www.ncbi.nlm.nih.gov). As such, the differential chromosomal segment is too large to identify the specific gene(s) underlying the above strain-differential effects. Further studies that involve the generation and detailed phenotyping of congenic substrains of SHR.1N, in which progressively smaller differential chromosomal segments are tested, are required. Nevertheless, the segment contains some interesting candidate genes. These include, for example, the peroxisome proliferator-activated receptor-δ gene, which encodes a FA-activated nuclear transcription factor shown previously to upregulate FA oxidation and energy uncoupling in adipose tissue (41).
Summary
The results of the present study suggest that the differential chromosomal segment of the congenic strain SHR.1N contains a gene (or several genes) that controls HFD-induced increases in body weight, fat pad weight, and adipocyte size, and that the gene may mediate these effects by regulating fat oxidation and whole body energy expenditure. As such, this molecular pathway may confer protection from diet-induced obesity. We also provide further evidence against the Tnfα gene being the gene responsible for the above effects. These results extend our previous findings demonstrating that the differential chromosomal segment controls HFD-induced increases in adiposity, glucose intolerance, and blood pressure, independently of food intake and physical activity (29). They also complement human studies suggesting that this locus plays a role in the pathogenesis of obesity and obesity-related diabetes mellitus and hypertension (7, 16, 25, 28, 36, 37).
GRANTS
The Canadian Institutes of Health Research (MOP-37915) and Research Project MSM 0021620807 supported this study. M. Pravenec is an international research scholar of the Howard Hughes Medical Institute.
Supplementary Material
Acknowledgments
The authors thank Tomas Paus and Catriona Syme for their helpful comments on the manuscript.
Address for reprint requests and other correspondence: Z. Pausová, Brain and Body Centre, Univ. of Nottingham, University Park, Nottingham NG7 2RD, UK (e-mail: zdenka.pausova@nottingham.ac.uk).
Footnotes
The online version of this article contains supplemental material.
REFERENCES
- 1.Ahima RS, Lazar MA. Adipokines and the peripheral and neural control of energy balance. Mol Endocrinol 22: 1023–1031, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev 84: 277–359, 2004. [DOI] [PubMed] [Google Scholar]
- 3.Cawthorn WP, Sethi JK. TNF-alpha and adipocyte biology. FEBS Lett 582: 117–131, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cederberg A, Gronning LM, Ahren B, Tasken K, Carlsson P, Enerback S. FOXC2 is a winged helix gene that counteracts obesity, hypertriglyceridemia, and diet-induced insulin resistance. Cell 106: 563–573, 2001. [DOI] [PubMed] [Google Scholar]
- 5.Chavey C, Mari B, Monthouel MN, Bonnafous S, Anglard P, Van Obberghen E, Tartare-Deckert S. Matrix metalloproteinases are differentially expressed in adipose tissue during obesity and modulate adipocyte differentiation. J Biol Chem 278: 11888–11896, 2003. [DOI] [PubMed] [Google Scholar]
- 6.Cornelius P, Enerback S, Bjursell G, Olivecrona T, Pekala PH. Regulation of lipoprotein lipase mRNA content in 3T3-L1 cells by tumour necrosis factor. Biochem J 249: 765–769, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fernandez-Real JM, Gutierrez C, Ricart W, Casamitjana R, Fernandez-Castaner M, Vendrell J, Richart C, Soler J. The TNF-alpha gene Nco I polymorphism influences the relationship among insulin resistance, percent body fat, and increased serum leptin levels. Diabetes 46: 1468–1472, 1997. [DOI] [PubMed] [Google Scholar]
- 8.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA 287: 356–359, 2002. [DOI] [PubMed] [Google Scholar]
- 9.Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest 114: 1752–1761, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gardel ML, Shin JH, MacKintosh FC, Mahadevan L, Matsudaira P, Weitz DA. Elastic behavior of cross-linked and bundled actin networks. Science 304: 1301–1305, 2004. [DOI] [PubMed] [Google Scholar]
- 11.Guerra C, Koza RA, Yamashita H, Walsh K, Kozak LP. Emergence of brown adipocytes in white fat in mice is under genetic control. Effects on body weight and adiposity. J Clin Invest 102: 412–420, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo KY, Halo P, Leibel RL, Zhang Y. Effects of obesity on the relationship of leptin mRNA expression and adipocyte size in anatomically distinct fat depots in mice. Am J Physiol Regul Integr Comp Physiol 287: R112–R119, 2004. [DOI] [PubMed] [Google Scholar]
- 13.Hansen JB, Kristiansen K. Regulatory circuits controlling white versus brown adipocyte differentiation. Biochem J 398: 153–168, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hara-Chikuma M, Sohara E, Rai T, Ikawa M, Okabe M, Sasaki S, Uchida S, Verkman AS. Progressive adipocyte hypertrophy in aquaporin-7-deficient mice: adipocyte glycerol permeability as a novel regulator of fat accumulation. J Biol Chem 280: 15493–15496, 2005. [DOI] [PubMed] [Google Scholar]
- 15.Hara-Chikuma M, Verkman AS. Aquaporin-3 functions as a glycerol transporter in mammalian skin. Biol Cell 97: 479–486, 2005. [DOI] [PubMed] [Google Scholar]
- 16.Herrmann SM, Ricard S, Nicaud V, Mallet C, Arveiler D, Evans A, Ruidavets JB, Luc G, Bara L, Parra HJ, Poirier O, Cambien F. Polymorphisms of the tumour necrosis factor-alpha gene, coronary heart disease and obesity. Eur J Clin Invest 28: 59–66, 1998. [DOI] [PubMed] [Google Scholar]
- 17.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science 259: 87–91, 1993. [DOI] [PubMed] [Google Scholar]
- 18.Huber J, Loffler M, Bilban M, Reimers M, Kadl A, Todoric J, Zeyda M, Geyeregger R, Schreiner M, Weichhart T, Leitinger N, Waldhausl W, Stulnig TM. Prevention of high-fat diet-induced adipose tissue remodeling in obese diabetic mice by n-3 polyunsaturated fatty acids. Int J Obes (Lond) 31: 1004–1013, 2007. [DOI] [PubMed] [Google Scholar]
- 19.Jaffe AB, Hall A. Rho GTPases: biochemistry and biology. Annu Rev Cell Dev Biol 21: 247–269, 2005. [DOI] [PubMed] [Google Scholar]
- 20.Jbilo O, Ravinet-Trillou C, Arnone M, Buisson I, Bribes E, Peleraux A, Penarier G, Soubrie P, Le Fur G, Galiegue S, Casellas P. The CB1 receptor antagonist rimonabant reverses the diet-induced obesity phenotype through the regulation of lipolysis and energy balance. FASEB J 19: 1567–1569, 2005. [DOI] [PubMed] [Google Scholar]
- 21.Kopecky J, Clarke G, Enerback S, Spiegelman B, Kozak LP. Expression of the mitochondrial uncoupling protein gene from the aP2 gene promoter prevents genetic obesity. J Clin Invest 96: 2914–2923, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kus V, Prazak T, Brauner P, Hensler M, Kuda O, Flachs P, Janovska P, Medrikova D, Rossmeisl M, Jilkova Z, Stefl B, Pastalkova E, Drahota Z, Houstek J, Kopecky J. Induction of muscle thermogenesis by high-fat diet in mice: association with obesity-resistance. Am J Physiol Endocrinol Metab 295: E356–E367, 2008. [DOI] [PubMed] [Google Scholar]
- 23.Listenberger LL, Ostermeyer-Fay AG, Goldberg EB, Brown WJ, Brown DA. Adipocyte differentiation-related protein reduces the lipid droplet association of adipose triglyceride lipase and slows triacylglycerol turnover. J Lipid Res 48: 2751–2761, 2007. [DOI] [PubMed] [Google Scholar]
- 24.Mokdad AH, Ford ES, Bowman BA, Dietz WH, Vinicor F, Bales VS, Marks JS. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA 289: 76–79, 2003. [DOI] [PubMed] [Google Scholar]
- 25.Norman RA, Bogardus C, Ravussin E. Linkage between obesity and a marker near the tumor necrosis factor-alpha locus in Pima Indians. J Clin Invest 96: 158–162, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pape ME, Kim KH. Effect of tumor necrosis factor on acetyl-coenzyme A carboxylase gene expression and preadipocyte differentiation. Mol Endocrinol 2: 395–403, 1988. [DOI] [PubMed] [Google Scholar]
- 27.Pausova Z From big fat cells to high blood pressure: a pathway to obesity-associated hypertension. Curr Opin Nephrol Hypertens 15: 173–178, 2006. [DOI] [PubMed] [Google Scholar]
- 28.Pausova Z, Deslauriers B, Gaudet D, Tremblay J, Kotchen TA, Larochelle P, Cowley AW, Hamet P. Role of tumor necrosis factor-alpha gene locus in obesity and obesity-associated hypertension in French Canadians. Hypertension 36: 14–19, 2000. [DOI] [PubMed] [Google Scholar]
- 29.Pausova Z, Sedova L, Berube J, Hamet P, Tremblay J, Dumont M, Gaudet D, Pravenec M, Kren V, Kunes J. Segment of rat chromosome 20 regulates diet-induced augmentations in adiposity, glucose intolerance, and blood pressure. Hypertension 41: 1047–1055, 2003. [DOI] [PubMed] [Google Scholar]
- 30.Pausova Z, Tremblay J, Hamet P. Gene-environment interactions in hypertension. Curr Hypertens Rep 1: 42–50, 1999. [DOI] [PubMed] [Google Scholar]
- 31.Pfaffl MW A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29: e45, 2001. [DOI] [PMC free article] [PubMed]
- 32.Pravenec M, Klir P, Kren V, Zicha J, Kunes J. An analysis of spontaneous hypertension in spontaneously hypertensive rats by means of new recombinant inbred strains. J Hypertens 7: 217–221, 1989. [PubMed] [Google Scholar]
- 33.Rosen ED The transcriptional basis of adipocyte development. Prostaglandins Leukot Essent Fatty Acids 73: 31–34, 2005. [DOI] [PubMed] [Google Scholar]
- 34.Rosen ED, Spiegelman BM. Adipocytes as regulators of energy balance and glucose homeostasis. Nature 444: 847–853, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seale P, Bjork B, Yang W, Kajimura S, Chin S, Kuang S, Scime A, Devarakonda S, Conroe HM, Erdjument-Bromage H, Tempst P, Rudnicki MA, Beier DR, Spiegelman BM. PRDM16 controls a brown fat/skeletal muscle switch. Nature 454: 961–967, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sookoian S, Garcia SI, Gianotti TF, Dieuzeide G, Gonzalez CD, Pirola CJ. The G-308A promoter variant of the tumor necrosis factor-alpha gene is associated with hypertension in adolescents harboring the metabolic syndrome. Am J Hypertens 18: 1271–1275, 2005. [DOI] [PubMed] [Google Scholar]
- 37.Sookoian SC, Gonzalez C, Pirola CJ. Meta-analysis on the G-308A tumor necrosis factor alpha gene variant and phenotypes associated with the metabolic syndrome. Obes Res 13: 2122–2131, 2005. [DOI] [PubMed] [Google Scholar]
- 38.Spiegelman BM, Farmer SR. Decreases in tubulin and actin gene expression prior to morphological differentiation of 3T3 adipocytes. Cell 29: 53–60, 1982. [DOI] [PubMed] [Google Scholar]
- 39.Timmons JA, Wennmalm K, Larsson O, Walden TB, Lassmann T, Petrovic N, Hamilton DL, Gimeno RE, Wahlestedt C, Baar K, Nedergaard J, Cannon B. Myogenic gene expression signature establishes that brown and white adipocytes originate from distinct cell lineages. Proc Natl Acad Sci USA 104: 4401–4406, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Um SH, Frigerio F, Watanabe M, Picard F, Joaquin M, Sticker M, Fumagalli S, Allegrini PR, Kozma SC, Auwerx J, Thomas G. Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature 431: 200–205, 2004. [DOI] [PubMed] [Google Scholar]
- 41.Wang YX, Lee CH, Tiep S, Yu RT, Ham J, Kang H, Evans RM. Peroxisome-proliferator-activated receptor delta activates fat metabolism to prevent obesity. Cell 113: 159–170, 2003. [DOI] [PubMed] [Google Scholar]
- 42.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 112: 1796–1808, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu H, Uysal KT, Becherer JD, Arner P, Hotamisligil GS. Altered tumor necrosis factor-alpha (TNF-alpha) processing in adipocytes and increased expression of transmembrane TNF-alpha in obesity. Diabetes 51: 1876–1883, 2002. [DOI] [PubMed] [Google Scholar]
- 44.Yamada T, Katagiri H, Ishigaki Y, Ogihara T, Imai J, Uno K, Hasegawa Y, Gao J, Ishihara H, Niijima A, Mano H, Aburatani H, Asano T, Oka Y. Signals from intra-abdominal fat modulate insulin and leptin sensitivity through different mechanisms: neuronal involvement in food-intake regulation. Cell Metab 3: 223–229, 2006. [DOI] [PubMed] [Google Scholar]
- 45.Zabel BA, Ohyama T, Zuniga L, Kim JY, Johnston B, Allen SJ, Guido DG, Handel TM, Butcher EC. Chemokine-like receptor 1 expression by macrophages in vivo: regulation by TGF-beta and TLR ligands. Exp Hematol 34: 1106–1114, 2006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.