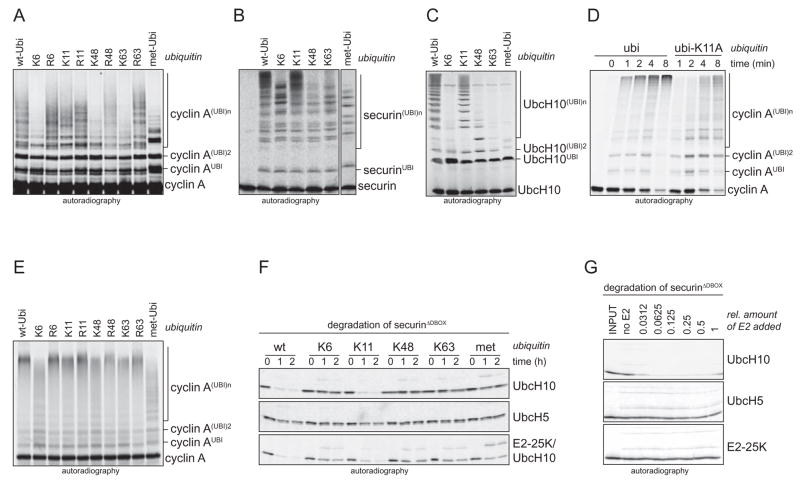
Figure 2. APC/CCdh1 and UbcH10 preferentially assemble K11-linked chains in vitro.
A. APC/CCdh1 and UbcH10 assemble chains on cyclin A using ubi-K11, but no other single-lysine mutant. APC/CCdh1 and UbcH10 were incubated with E1 and ubiquitin mutants. B. APC/CCdh1 and UbcH10 assemble chains on the processive substrate securin using ubi-K11, but no other tested single-lysine mutant. C. UbcH10-autoubiquitination by APC/CCdh1 is supported by ubi-K11 while other single-lysine mutants are much less efficient. D. The formation of ubiquitin chains by APC/CCdh1 and UbcH10 on cyclin A is delayed, if K11 of ubiquitin is mutated. The kinetics of chain formation on cyclin A by APC/CCdh1 and UbcH10 in the presence of wt-ubi and ubi-K11A was analyzed by autoradiography. E. APC/CCdh1 and UbcH5c form ubiquitin chains on cyclin A linked through K11, K48, and K63. APC/CCdh1 and UbcH5c were incubated with E1 and ubiquitin mutants. Similar results were obtained with UbcH5a. F. UbcH5 is less efficient in promoting the degradation of securinΔD in G1-extracts. G1-extracts were supplemented with ubiquitin mutants and UbcH10 (upper panel), UbcH5c (lower panel), and a combination of UbcH10 and E2-25K (lower panel). Degradation of radiolabeled securinΔD was monitored by autoradiography. G. UbcH10 is more potent in triggering degradation of securinΔD in G1-extracts over a wide range of concentrations. Three E2s, UbcH10, UbcH5c, and E2-25K, were titrated in G1-extracts. Reactions were analyzed after 60 min for securinΔD-levels by autoradiography.