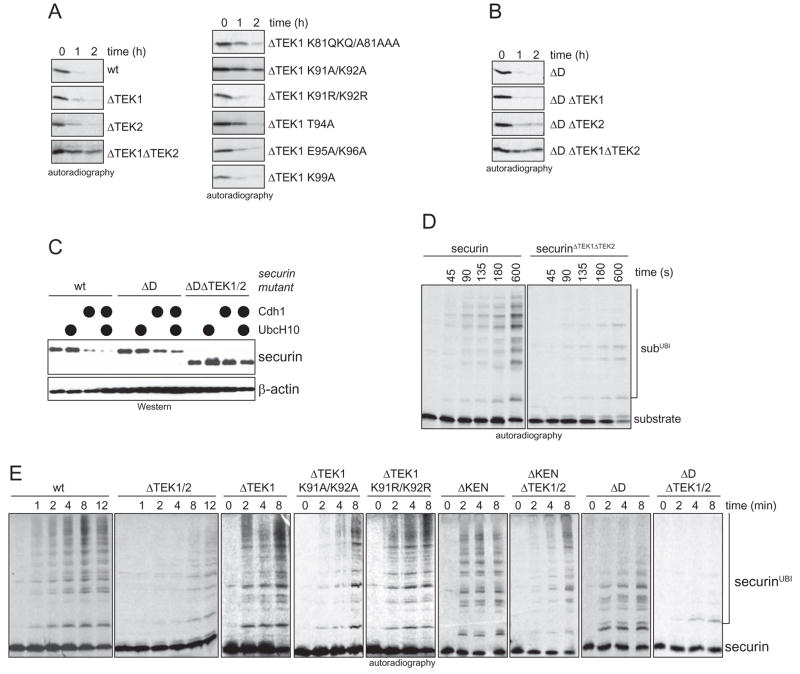
Figure 6. The TEK-box in securin is required for efficient UbcH10-dependent ubiquitination and degradation.
A. Deletion of both TEK-boxes stabilizes securin in a UbcH10-dependent degradation assay. Degradation of indicated securin-mutants in extracts of quiescent T24 cells supplemented with UbcH10 was monitored by autoradiography. B. Deletion of both TEK-boxes impairs the UbcH10-dependent degradation of securinΔD. Degradation of securinΔD-mutants in G1-extracts supplemented with UbcH10, as monitored by autoradiography. C. Deletion of both TEK-boxes stabilizes the APC/CCdh1-substrate securinΔD in cells. The APC/C-dependent degradation of the indicated securin mutants was triggered in 293T cells by co-expression of Cdh1, mycUbcH10, or both (●). Expression levels were analyzed by Western blotting. D. Deletion of both TEK-boxes delays modification of securin lysine residues. The kinetics of monoubiquitination of radiolabeled securin or securinΔTEK1/2 by APC/CCdh1 and UbcH10 was monitored in the presence of methylubiquitin by autoradiography. E. Deletion of both TEK-boxes delays the onset of chain formation on securin by APC/CCdh1 and UbcH10. Radiolabeled securin and the indicated mutants were incubated with APC/CCdh1 and UbcH10 in the presence of ubiquitin, and analyzed by autoradiography.