Table 2.
Aminolysis of unactivated esters
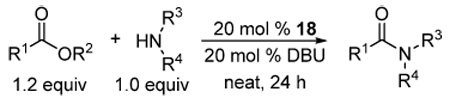 | ||||
|---|---|---|---|---|
| entry | ester | amine | Temp °C | % yielda |
| 1 | MeCO2Me | PhCH2NH2 | 23 | 83 (3) |
| 45 | 94 (4) | |||
| 2 | PhCO2Me | PhCH2NH2 | 23 | 26b (2) |
| 70 | 92 (3) | |||
| 3 | Me(CH2)6CO2Me | PhCH2NH2 | 23 | 43b (1) |
| 70 | 94 (2) | |||
| 4 | i-PrCO2Me | PhCH2NH2 | 23 | 23b (nd) |
| 70 | 62 (2) | |||
| 5 | MeCH(OH)CO2Me | PhCH2NH2 | 23c | 52b (8) |
| 23 | 84 (50) | |||
| 6 | MeCO2Et | PhCH2NH2 | 23 | 58 b (1) |
| 70 | 91 (7) | |||
| 7 | γ−butyrolactone | PhCH2NH2 | 23c | 84 (5) |
| 8 | Me(CH2)6CO2Me | piperidine | 23 | 2b (nd) |
| 95 | 77 (2) | |||
| 9 | Me(CH2)6CO2Me | PhCH(Me)NH2 | 23 | 1 b (nd) |
| 95 | 71 (5) | |||
| 10 | Me(CH2)6CO2Me | c-C6H11NH2 | 23 | 3b (nd) |
| 95 | 84 (2) | |||
| 11 | (L)-Phe-OMe | 90 | 79 (7) | |
Isolated yield is given, unless specified otherwise. % Conversion by 1H NMR in the absence of 18 is given in parentheses.
Yield estimated by 1H NMR
Carried out in CDCl3 at 1.0 M of amine
