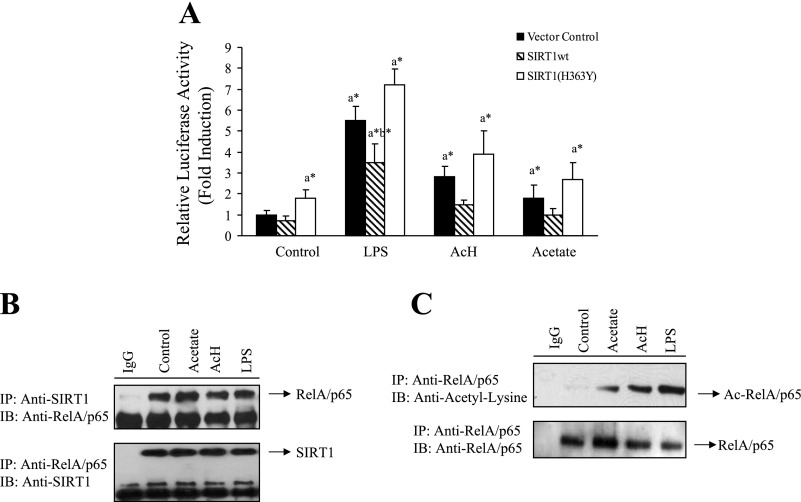
Fig. 4.
LPS-, AcH-, or acetate-mediated inhibition of SIRT1 was associated with increased acetylation of RelA/p65 and stimulated NF-κB transcriptional activity. A: RKC1 cells were transiently transfected with a NF-κB-responsive reporter-3xκB luciferase (10 μg) and expression plasmids (5 μg/each) of SIRT1wt or SIRT1(H363Y) and β-galactosidase (2 μg; internal control). LPS (100 μM), AcH, (100 μM), or acetate (20 mM) was added for 18 h. At 48 h after transfection, cells were harvested, and luciferase and β-galactosidase activities were determined as described in materials and methods. B: RKC1 cells were starved in serum-free DMEM overnight and treated with LPS (100 ng/ml), AcH, (100 μM) or acetate (20 mM) for 18 h. Cell extracts were immunoprecipitated (IP) with an antibody to SIRT1 or RelA/65 and immunoblotted (IB) with an anti-SIRT1 or an anti-RelA/65 antibody. C: RKC1 cells were treated as described in A. Immunoprecipitations were performed with an antibody to RelA/p65 and immunoblotted with an acetyl-lysine or a RelA/p65 antibody. All data are means ± SE for 3 or 4 replications. *P < 0.05 by 1-way ANOVA. aSignificantly different compared with control group. bSignificantly different compared with LPS-treated vector control group.