Abstract
Context
Corticotropin-releasing hormone (CRH), through the hypothalamic pituitary adrenal (HPA) axis and other brain stress systems, is involved in the emotional dysregulation associated with cocaine dependence. Little is known about the response of cocaine-dependent individuals to CRH administration.
Objective
The primary objective was to examine the HPA axis, subjective and physiologic response to CRH in cocaine-dependent individuals and controls.
Design
Case-control study
Setting
Subjects were admitted to a General Clinical Research Center (GCRC) for testing and abstinence verified with urine drug screening.
Participants
Participants were control males (n=23), control females (n=24), cocaine-dependent males (n=28), and cocaine-dependent females (n=25). Individuals with dependence on other substances (except caffeine, nicotine) or with major depression, PTSD, bipolar, psychotic and eating disorders were excluded.
Intervention
Subjects received i.v. CRH (1ug/kg).
Main Outcome Measures
Primary outcomes included plasma ACTH and cortisol, heart rate, and subjective measurements.
Results
Cocaine-dependent individuals exhibited higher stress (P < 0.001) and craving to CRH compared to controls. A positive correlation (rs=.51, P=0.0002) between stress and craving was found in cocaine dependent subjects. CRH elevated heart rates in all groups, however cocaine dependent females, demonstrated a significantly higher heart rate at all time points (P=0.05). Women had higher cortisol response to CRH (P=0.028). No effect of cocaine status was observed. ACTH response to CRH was independent of gender and cocaine. Cortisol and ACTH were positively correlated in the controls and cocaine-dependent males, but not in cocaine-dependent females (rs = 0.199; P = 0.4).
Conclusion
There is an increased subjective and heart rate response to CRH and a relationship between stress and craving in cocaine-dependent individuals. The lack of difference in HPA axis response between the cocaine and control groups suggests that the heart rate and subjective responses in the cocaine group may be mediated by sensitization of non-hypothalamic stress-responsive CRH systems.
Introduction
It has been postulated that dysregulation of HPA axis, brain reward, and corticotropin-releasing hormone (CRH)-involved stress systems is a critical part of the allostatic changes associated with the transition from drug use to dependence as the organism attempts to maintain reward function stability through changes in reward and stress system neurocircuitry1. CRH is thought to play a critical role in the emotional dysregulation associated with cocaine dependence and relapse through actions on both the HPA axis and brain stress systems in the extended amygdala2. The HPA axis is activated during binge cocaine use 3–6 and contributes to the activation of the brain reward systems 7, 8. Chronic cocaine use is associated with attenuation of the HPA response in animal models 2, 9 and the clinical laboratory setting 10, 11. Interestingly, there are CRH receptors within the processive limbic circuitry which are essential to determining the salience of environmental stressors, suggesting that CRH may play a role in stress-induced relapse 12. Animal models demonstrate that escalation in cocaine intake produces activation of CRH in the extended amygdala which is particularly evident during withdrawal and may play a prominent role in the maintenance of cocaine self-administration 2. In animal models of relapse, administration of CRH or exposure to a variety of stressors facilitates reinstatement of self-administration of drugs of abuse and this effect is blocked by CRH antagonists 13.
There are important gender differences in stress response and HPA axis function that may play a role in gender differences in relapse. Women consume cocaine using more addictive routes and progress from occasional drug use to dependence faster than men 14, 15. In addition, cocaine-dependent women have more affective and anxiety disorders as compared to men 16 and these comorbidities are also associated with HPA axis and stress response dysregulation. The brain circuitry (including the amygdala and hippocampus) underlying cognitive processing of stress is sexually dimorphic in both humans and laboratory animals 17. In addition to circuitry differences, hormonal regulation contributes to sexual dimorphism in stress responses 18, with both estrogen and progesterone acting as potent modulators of HPA axis stress regulation 19–21.
The primary focus of the present study was to investigate the HPA axis, physiologic and subjective response to a CRH infusion in cocaine-dependent men and women as compared to a matched control group. Since this study is a part of a larger grant focused on gender differences in substance use disorders, gender differences in response were studied.
Methods
Subjects
The sample consisted of 100 participants. Demographic characteristics are presented in Table 1. Subjects were recruited primarily via media advertisements over a 48-month period. Data for the present study were drawn from a larger study on gender differences in stress and cue reactivity among cocaine dependent and control participants. Written informed consent was obtained before study assessments were administered. All procedures were conducted in accordance with Good Clinical Practice Guidelines and the Declaration of Helsinki, and received Institutional Review Board (IRB) approval. General exclusion criteria included (1) current major depressive and PTSD; (2) history of or current medical conditions that might interfere with safe conduct of the study or affect HPA activity; (3) history of or current psychotic, eating, or bipolar affective disorders; (4) synthetic glucocorticoid or exogenous steroid therapy within one month of testing; (5) current benzodiazepine, antipsychotic, b-blocker and other medication use that might interfere with HPA axis activity or psychophysiologic measurement; (6) pregnancy, nursing, or ineffective means of birth control; (7) body mass index ≥ 35; or (8) DSM-IV criteria for substance dependence except caffeine, nicotine or marijuana within the past 60 days.
Table 1.
Subject Demographics by Group
| Subject Variable | Control Males (n=23) | Control Females (n=24) | Cocaine Dependent Males (n=28) | Cocaine Dependent Females (n=25) | P value |
|---|---|---|---|---|---|
| Age, mean (SD), y | 31.7 (10.3) | 39.8 (11.5) | 38.0 (11.2) | 39.0 (10.4) | NS |
| Education, % Some College | 86 | 87 | 67 | 40 | 0.001 |
| Employment, % Employed | 64 | 92 | 59 | 40 | 0.001 |
| Race, % Caucasian | 70 | 58 | 50 | 50 | NS |
| Marital Status, % Married | 14 | 29 | 7 | 12 | NS |
| Smoking Status, % Smokers | 65 | 50 | 79 | 80 | NS |
NS Indicates not significant (P ≥ 0.05)
Assessment
Subjects meeting preliminary screen criteria were evaluated for study eligibility with the SCID-IV, which permits accurate diagnosis of lifetime and current psychiatric disorders using DSM-IV criteria 22. Substance use in the ninety days prior to study enrollment and throughout the study period was assessed using the Time-Line Follow-Back 23. A medical history and physical examination, including electrocardiogram, were completed to assess for medical exclusions. Menstrual history was obtained for female subjects. While we attempted to schedule all female subjects for testing during the luteal phase of the menstrual cycle, this did not prove to be feasible. Delaying the scheduling of testing, particularly for cocaine-dependent women, led to a drop-out rate that made it impossible to complete the study in a reasonable time-frame. Following baseline assessments, participants were scheduled to complete the laboratory procedure.
CRH administration
All laboratory procedures were conducted at the General Clinical Research Center (GCRC) of the Medical University of South Carolina. Subjects were admitted to an inpatient hospital unit at approximately 2000h the evening prior to testing in order to control extraneous variables that could affect stress reactivity (e.g., nutrition, caffeine intake, sleep, nicotine use). Subjects were required to abstain from alcohol or other substance use (except nicotine and caffeine) for a minimum of three days prior to testing. Abstinence was assessed using self-report, urine drug screen (Roche Diagnostics), and breathalyzer tests (AlcoSensor III, Intoximeters, Inc). Subjects dependent on nicotine were provided with a nicotine patch (n= 66).
On the morning following admission, subjects were provided a standard breakfast at 0830h. They were allowed to engage in sedentary activities on the unit (e.g., reading magazines, watching TV) until testing. At 1150h, an indwelling intravenous catheter was inserted in the forearm of the non-dominant hand. At 1200h subjects were given a standard lunch. Following lunch, the participants were connected to electrodes for heart rate and to an intermittently inflatable blood pressure cuff. Between 1340h and 1400h, the subjects completed additional testing procedures. Specifically, subjects were exposed either to cocaine-related paraphernalia for a period of approximately 10 minutes, or they completed the Trier Social Stress Task. Data from these tasks will be presented in a separate manuscript. Neuroendocrine, physiologic, and subjective outcomes returned to baseline levels within 20 minutes of the Trier. Subjects were allowed a rest period prior to CRH administration.
Beginning at 1640h, two baseline assessments of subjective, heart rate, and neuroendocrine parameters were obtained 10-minutes apart to provide for a stable baseline index for challenge response comparisons. Pre-session subjective scales included the Craving/Distress/Mood Scale, a modification of the Within Session Rating Scale designed to rapidly assess craving and other mood feeling states (including stress) during the test session 24. This 100-mm visual 10-point Likert scale is anchored with adjectival modifiers (“not at all” to “extremely”). This scale was also utilized at each of the post-task assessment time points.
At 1700h, CRH (1ug/kg to a maximum dose of 100ug; provided by Ferring Pharmaceuticals) was administered via IV push over a one-minute period. Immediately following CRH administration, subjective ratings and heart rate measurements were obtained and blood was collected for neuroendocrine assay (ACTH and cortisol). Neuroendocrine samples, subjective ratings, and physiologic measurements were further assessed at 5-minute, 30-minute, 60-minute, and 120-minute intervals post task. Blood samples were collected in EDTA-prepared tubes and immediately placed on ice. Plasma was obtained by centrifugation under refrigeration and the serum sample frozen at −70° C until assayed in duplicate. Allegro HS-ACTH system (Nichols Institute Diagnostics), which has an intra-assay c.r. of 6% with a sensitivity of 1 pg/ml, was used for ACTH assays. Cortisol was assayed using the Roche Diagnostic Elecsys 2010 immunoassay analyzer and kits based on an electrochemiluminescence competitive immunoassay having a functional sensitivity of 0.29 µg/dL and intra-assay reproducibility of less than 2%. GCRC personnel collected all samples, and Rockefeller University personnel performed the analysis. Heart rate was collected via three electrodes along the bottom of the participant’s ribcage, bicep, and collar bone.
Following completion of data collection, subjects remained in the hospital overnight and completed additional tasks the following day. The subjects were debriefed and compensated for their participation prior to discharge from the GCRC.
Statistical Analysis
Subjective measures (stress and craving) were summarized by calculating area under the curve (AUC) using the trapezoid rule. Experimental group differences in median AUC were conducted using the nonparametric Kruskal-Wallis test.
Physiological (heart rate) and neuroendocrine (ACTH, cortisol) outcomes were analyzed using covariance pattern models to account for repeated longitudinal measurements taken on each subject. To account for non-constant variability among the groups, the estimated covariance matrix was allowed to vary by gender or cocaine status, as appropriate. These analyses were performed via the mixed procedure (SAS/STAT software, Version 9.1.3 of the SAS System for Windows. Copyright © 2002–2003 SAS Institute Inc. SAS and all other SAS Institute Inc. product or service names are registered trademarks or trademarks of SAS Institute Inc., Cary, NC, USA). Non-normality of the outcomes and residuals was addressed via log10 transformations. Prior to the repeated measures analyses, the effects of gender and cocaine status on each of the baseline responses were assessed via non-parametric Wilcoxon rank-sum tests. Where baseline differences in physiological or neuroendocrine measures were found, the baseline measure was included as a covariate in that model. All longitudinal analyses controlled for smoking status, race, and age.
Peak change in subjective, physiological, and neuroendocrine outcomes were calculated as the percent change in response over baseline: (max response – baseline response) / baseline response × 100. Peak change was analyzed using a multivariable linear model and transformations were used where appropriate. In multivariable analysis, the covariates (smoking status, race and age) were controlled for where possible confounding was suspected. For multiple comparisons that were not part of the hypothesis a priori, the Bonferroni correction was used.
To test our hypothesis of HPA dysregulation, we examined Spearman’s rank correlation coefficient (rs) of peak change in physiological, neuroendocrine, and subjective outcomes, by experimental group. Where descriptive statistics are presented, they represent the mean + standard error. P-values less than 0.05 were considered significant findings.
RESULTS
Subject Demographic and Descriptive Data
In Table 1, subject demographic and descriptive data by cocaine status and gender are displayed. While there were no significant differences in age, race, marital or smoking status, there were significant differences in education and employment with the cocaine group demonstrating significantly lower educational (P < 0.001) and employment levels (P < 0.001). Four control females and two cocaine-dependent females met criteria for social phobia or generalized anxiety disorder. Two cocaine-dependent males and one cocaine-dependent female met criteria for marijuana dependence.
Subjective Measures
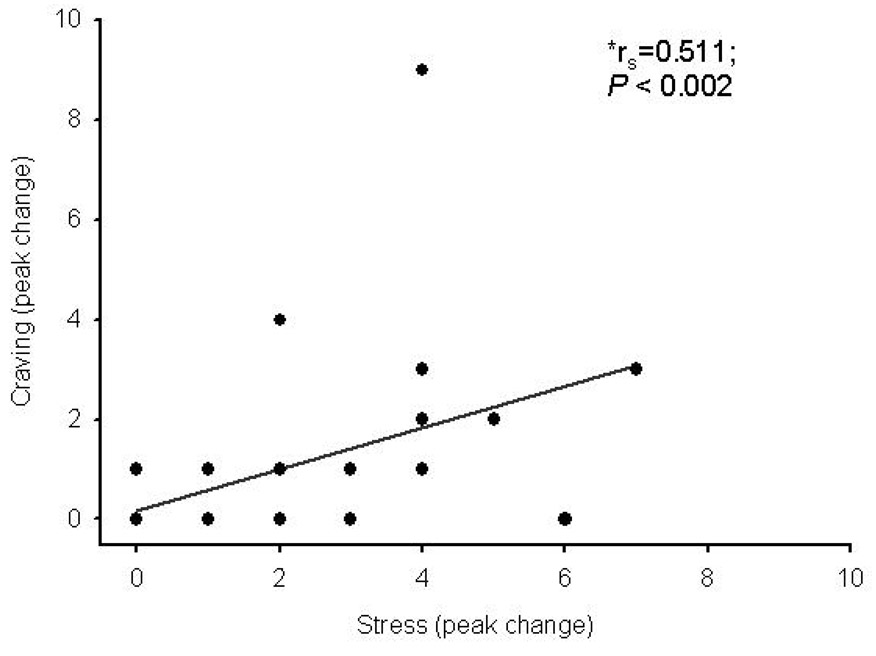
While some of the control subjects reported an increase in subjective stress following CRH administration (12/45), both the number of responders (21/48) and the magnitude of subjective stress response was significantly higher in the cocaine-dependent individuals than in the control group (Figure 1A). This response was significantly more robust in the cocaine group as measured across all time points by AUC (P < 0.001). There were no gender differences in this response. Cocaine-dependent subjects also experienced craving after CRH administration, while, as would be expected, the control group subjects did not (Figure 1B). A significant correlation was found between peak stress and peak craving in cocaine-dependent individuals (rs=0.511; P <0.0002) (Figure 2).
Figure 1.
Subjective stress and craving in response to CRH. Stress (A) and craving (B) data are presented as group means (+se) from control males (closed triangles), control females (open triangles), cocaine dependent males (closed circles), and cocaine dependent females (open circles) during baseline (−20) and following CRH administration (time 0).
Figure 2.
Positive correlation between peak stress and peak craving following CRH administration in cocaine dependent subjects. *Denotes Spearman’s rank correlation coefficient (rs). P < 0.05 denotes a significance correlation between peak change in stress and peak change in craving.
Heart Rate
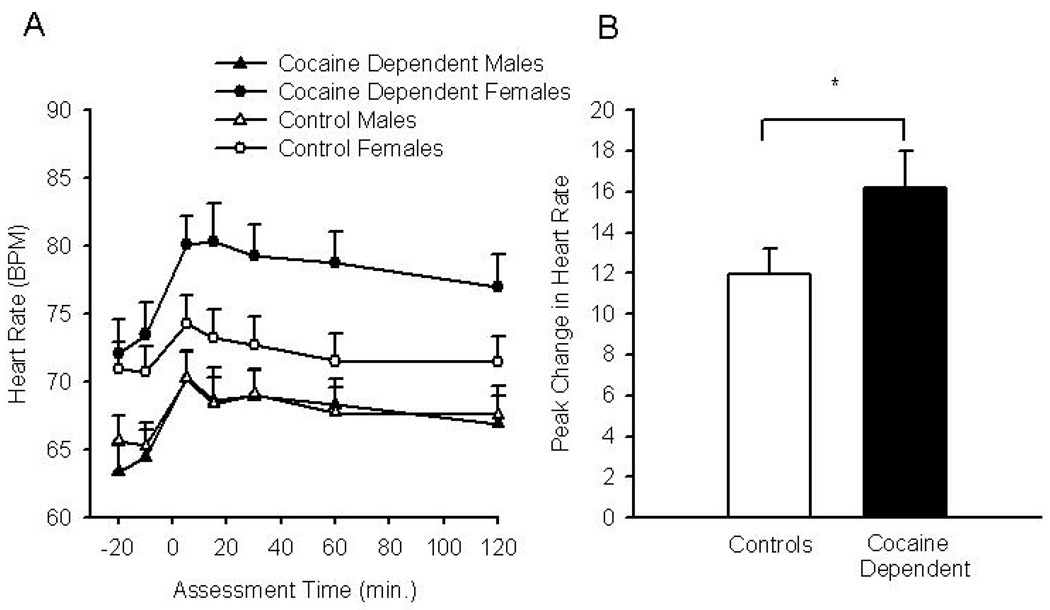
Heart rate was increased following CRH administration across all groups (F4,89 = 6.37, P = 0.0002) (Figure 3A). There was a significant difference in baseline heart rate between men and women (P <0.0001), with women demonstrating a higher heart rate response to CRH as compared to men (71.5 + 9.5 versus 64.4 + 9.9). As a result, baseline heart rate was included as a covariate in the subsequent analysis. Cocaine-dependent females had significantly higher heart rates over the course of the study as compared to the other three groups (F1,88 = 3.87, P = 0.05) (Figure 3A). A peak change analysis for CRH heart rate responders was conducted. Responders were defined as having a greater than 0 percent change over baseline heart rate. There were 87 responders (control males, n=22; control females, n=19; cocaine dependent males, n=26; cocaine dependent females, n=20). In a multivariable analysis of responders, cocaine use was an independent marginal predictor of higher heart rate response (cocaine: X2(1) =3.66, P = 0.06) (Figure 3B).
Figure 3.
Heart rate response to CRH. Mean heart rate response from control males (closed triangles), control females (open triangles), cocaine dependent males (closed circles), and cocaine dependent females (open circles) during baseline (−20 and −10) and following CRH administration Data are presented as group means +se (A). Responder analysis comparing peak change in heart rate between non-dependent controls (males and females) and cocaine dependent subjects (males and females) (B). Data are presented as group means +se, *Denotes a significant difference between the cocaine dependent and control groups.
ACTH
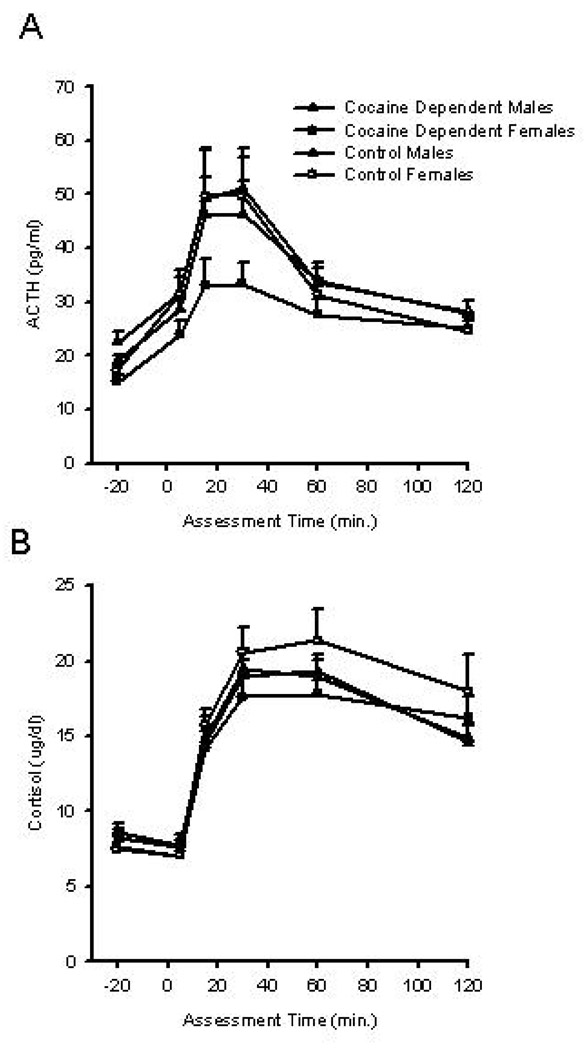
ACTH levels were increased across all groups following CRH administration (F4, 84.4 = 2.63, P = 0.04) (Figure 4A). Women exhibited a significantly lower baseline ACTH than men (16.29 + 6.19 versus 20.49 + 7.35, P = 0.003), so baseline ACTH was included as a covariate in subsequent analyses. No group by time or gender by time interactions were found for ACTH response. Peak change in ACTH was analyzed using a linear model with a log10 transformation of peak change. A marginal significant cocaine x gender interaction (X2(1) =3.43, P = 0.06) was found indicating a greater peak change in ACTH levels following CRH administration in cocaine-dependent men as compared to cocaine-dependent women.
Figure 4.
Neuroendocrine response to CRH. Data are presented as group means (+se) from control males (closed triangles), control females (open triangles), cocaine dependent males (closed circles), and cocaine dependent females (open circles) during baseline (−20) and following CRH administration (time 0). Plasma ACTH was measured during baseline (−20) and at regular intervals following CRH infusion (time 0). (A) Plasma cortisol was measured during baseline (−20) and at regular intervals following CRH infusion (time 0). (B)
Cortisol
Cortisol levels were increased in all groups following CRH administration (F5,61= 118.99, P < 0.001) (Figure 4B). A group x time interaction was not observed indicating that the cortisol response to CRH infusion in cocaine-dependent individuals was similar to that of the control group. A significant gender x time interaction (F5,61 = 2.71, P = 0.028) in cortisol response was found indicating that the time course of the cortisol response differed between men and women. As can be seen (Figure 4B), both cocaine-dependent and control women have a higher cortisol at all time points following CRH administration as compared to men with elevated levels persisting at the 120 minute time measurement. An analysis of peak change in cortisol demonstrated a main effect of gender (X2(1) =4.97, P = 0.03).
ACTH Cortisol Peak Change Correlations
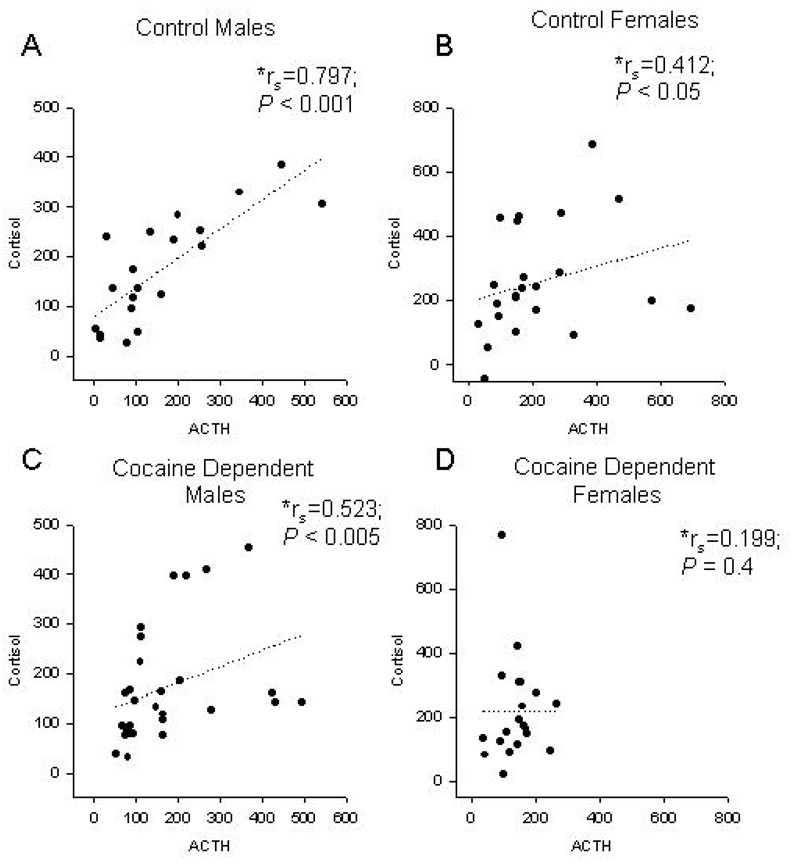
A significant positive correlation was observed between peak change in ACTH and cortisol in control males (rs=0.797; P < 0.001), control females (rs=0.412; P < 0.05), and cocaine dependent males (rs=0.523; P < 0.005). However, there was no association between ACTH and cortisol in cocaine dependent females (rs =0.199; P = 0.4) (Figure 5).
Figure 5.
HPA dysregulation in cocaine dependent females. Correlation between peak change in plasma cortisol and peak change in plasma ACTH in response to CRH administration in control males (A) control females (B) cocaine dependent males (C) and cocaine dependent females (D). Data were analyzed using Spearman’s rank correlation coefficients (*rs). P < 0.05 denotes a significance correlation between peak change in ACTH and peak change in cortisol.
Past and Future Cocaine Use as Predictors of Response
The percent of days that cocaine was used in the 30 days before the start of the study and in the 30 days following participation in the study were analyzed to determine any relationship to subjective craving and stress or heart rate, ACTH, and cortisol response. Subjects reported using 35% of the 30 days prior to the study and 18% of the 30 days following the study. There were no gender differences in percent using days either before or after study participation. After adjusting for age, the percent of days used in the 30 days prior to the study was a significant predictor of the peak change in cortisol (P = 0.01), stress (P = 0.01), and craving (P < 0.001). The percent of days used in the 30 days following the study was a significant predictor of the peak change in stress (P < 0.03); while a trend (P < 0.1) was observed for peak change in craving and peak change in cortisol. No gender differences were found in these relationships.
Comments
In the present study, the subjective, physiologic and HPA axis responses to CRH in cocaine-dependent men and women were compared to that of a control group matched for gender, age, race and smoking status. The differences between the cocaine and control group in the subjective and heart rate response to CRH were striking. Both the number of responders and magnitude of subjective stress response to CRH in the cocaine-dependent individuals were significantly higher than in the control group across all time points. As previously discussed, with acute cocaine use, there is activation of the HPA axis. With prolonged use, there may be down-regulation of the HPA response, but activation of CRH systems in limbic circuitry, particularly the extended amygdala, which have been implicated in the behavioral responses to stressors 7, 9, 25, 26. A number of the brain sites hypothesized to be important for the behavioral effects of CRH are closely linked to norepinephrine systems including the locus coeruleus, bed nucleus of the stria terminalis and the central nucleus of the amygdala 27, 28. Of interest, there is data suggesting that norepinephrine release in these areas stimulates the release of CRH which would imply a powerful “feedforward” system 7 that might be a mechanism for sensitization of the stress response. The difference in the heart rate response to CRH between the cocaine and control group also provides support to the idea that CRH stimulation in the cocaine group may have been acting through preferentially sensitization of CRH -linked noradrenergic systems in the locus coeruleus which regulate the heart rate response to stress. Importantly, the CRH- noradrenergic interaction has been hypothesized as one of the mechanisms contributing to allostasis in the development of addiction 1, 2. The fact that there were not dramatic differences in the HPA axis response between the cocaine-dependent and control group suggests that the increased subjective and heart rate response to CRH in the cocaine-dependent group may be related to stimulation of CRH connections to the locus coeruleus and extended amygdala rather than stimulation of the HPA axis.
A substantial number of cocaine-dependent subjects (16/48) reported cocaine craving following CRH administration and the correlation between craving and stress was high emphasizing the importance of the stress-relapse connection on a neurobiologic and subjective level for cocaine-dependent individuals 29, 30. In an interesting series of studies, Sinha and colleagues 31, 32 reported that stress imagery and cocaine cues produce craving, anxiety ,HPA axis and sympatho-adreno-medullary responses that are similar to the arousal produced by cocaine itself in cocaine-dependent individuals. In the current study, CRH administration may have also produced responses similar to those experienced with cocaine administration for some cocaine-dependent individuals. Sinha and colleagues 32, 33 also found a good correlation between drug cue-induced and stress-imagery-induced stress and craving and that greater stress-induced, but not drug cue-induced craving was associated with shorter time to cocaine relapse after a laboratory session. They also found that both cortisol and ACTH peak response were positively associated with the amount of cocaine used per occasion in the 90-day period following study participation, but not associated with time to relapse or frequency of relapse. In the present study, the number of days using cocaine prior to and following study participation were predictive of peak stress and craving responses following CRH administration. A positive association was also found between peak cortisol change and the amount of cocaine use before study participation (P < 0.01) and a trend towards a relationship between cortisol change and cocaine use after study participation (P < 0.08). Together, these studies support the predictive validity of responses in laboratory paradigms designed to induce craving or stress and substance use in “real life” situations. In addition, these finding provide support for a positive relationship between HPA axis response and propensity to use cocaine. In contrast, several recent studies suggest that a blunted HPA axis response in alcohol-dependence is predictive of relapse 34, 35. However, the impact of chronic alcohol use on HPA axis function differs from that of chronic cocaine use 36, 37 and the fact that there is a relationship between HPA response and propensity to relapse in both disorders, regardless of the direction of change, might be the more important issue.
There was surprisingly little difference between the cocaine and control groups in the ACTH and cortisol response to CRH. While several studies have reported HPA axis dysregulation in cocaine-dependent individuals 36, others have noted no abnormalities in basal cortisol during early abstinence in cocaine-dependent individuals 38, 39. A previous study comparing HPA axis response to CRH (1.0 µg/kg) in a group of polysubstance abusing subjects to controls found lower cortisol and ACTH response in the polysubstance group 36, however this study included a heterogeneous group of individuals with substance use disorders including cocaine, alcohol, heroin and marijuana dependence while the population sampled in the current study primarily had cocaine dependence only. There are no other studies that we are aware of investigating the response to CRH in individuals with primary cocaine dependence and the impact of dependence on a variety of substances could explain the discrepancy in the findings between studies. The dose of CRH utilized may be a factor. In one study comparing two doses of CRH in methadone-maintained versus control subjects, no between group difference in ACTH response was found following low-dose CRH (0.5 µg/kg), but there was a greater ACTH response in the methadone-maintained group following high-dose CRH (2.0 µg /kg) as compared to the control group 40. In the present study, only one dose of CRH was explored (1.0 µg /kg) so it is possible that differences would have been found if a broader range of CRH dosing had been explored.
Gender differences in the activity of the HPA axis under basal conditions and in response to various challenges have been reported. In humans, basal cortisol levels are similar between men and women, but basal ACTH is significantly higher in men 41. A number of studies have found higher total plasma cortisol response to various challenge paradigms in women as compared to men, however this may reflect gender differences in the cortisol binding protein rather than higher free cortisol 42. The general gender findings of the present study are consistent with these studies. While there were no profound differences between the cocaine and control groups in the ACTH and cortisol response to CRH, there is some suggestion of greater disruption in cocaine-dependent women as compared to cocaine-dependent men. There was a trend towards lower ACTH response in cocaine-dependent women as compared to all other groups. In spite of the blunted ACTH response, the cortisol response in cocaine-dependent women was robust and did not decrease during the 120-minute follow-up period. Consistent with this finding, peak plasma ACTH was positively correlated with the peak plasma cortisol in the cocaine-dependent men and in both control groups, but not in cocaine-dependent women. The correlation between the ACTH and cortisol response in a well-regulated HPA axis response should be high and the lack of correlation in the cocaine-dependent women suggests dysregulation of the response. In addition, cocaine-dependent women demonstrated the greatest increase in heart rate and a prolonged heart rate response following CRH infusion as compared to all other groups. Taken together, these findings suggest greater sensitivity to the toxic effects of cocaine in women as compared to men. This is consistent with other studies demonstrating greater HPA dysregulation with chronic substance use in women as compared to men. Gianoulakis and colleagues 43 investigated the effect of chronic alcohol consumption on HPA axis activity as a function of alcohol intake, age and gender and found lower plasma ACTH levels in heavy drinkers which was more pronounced in women as compared to men. In a sub-analysis of data from the control group in the current study, female smokers evidenced a blunted cortisol response to CRH as compared to nonsmokers, whereas smoking status did not impact cortisol response in men 44. An increased vulnerability of women to adverse medical and psychosocial consequences of substance use has been well documented 15, 45–48 and women have been shown to advance more rapidly than men from initial to regular use 15, 49, 50. These clinical differences may be related to increased sensitivity to toxic effects of substances of abuse.
There are a number of important study limitations. There were unanticipated baseline differences in employment and educational status. However, important baseline variables such as age, gender, race and smoking status were controlled for and it is unlikely that employment or educational status impacted the primary study measures. The measurement points after CRH administration were limited and only one dose of CRH was tested, so some group differences might have been missed. In addition, it was not feasible to standardize menstrual cycle phase for women at the time of testing. Adding a saline infusion to serve as a control condition would have improved the study design as it is possible that the subjective response to CRH in the cocaine group was a placebo response. However, we would have expected a similar placebo response in control subjects. In addition, there was a difference in the heart rate response to CRH between the cocaine and control groups and peak stress and peak craving were correlated with percent of using days before and after the study. These findings make it less likely that the subjective response to CRH in the cocaine group was a placebo response.
In spite of these limitations, there were a number of intriguing findings. Cocaine-dependent subjects had a greater subjective and heart rate response without substantial difference in ACTH and cortisol response to CRH infusion. This may indicate differential stimulation of pathways in the extended amygdala and locus coeruleus by CRH in cocaine-dependent individuals. The robust relationship between stress and craving supports the role of stress in relapse in the clinical setting. Consistent with other studies, cocaine-dependent women appeared to be more sensitive to toxic effects of chronic use as evidenced by greater HPA axis dysregulation. Finally, the relationship between amount of cocaine use and stress/craving and cortisol response in the laboratory supports the validity of these laboratory paradigms in studying the neurobiologic underpinnings of relapse in individuals with substance use disorders.
Acknowledgements
Lisa Marie Jenkins, B.S. for technical assistance
Contributor Information
Kathleen T. Brady, Medical University of South Carolina, Psychiatry and Behavioral Neurosciences, Clinical Neurosciences Division, Charleston, SC USA
Aimee L. McRae, Medical University of South Carolina, Psychiatry and Behavioral Neurosciences, Clinical Neurosciences Division, Charleston, SC USA
Megan M. Moran-Santa Maria, Medical University of South Carolina, Psychiatry and Behavioral Neurosciences, Clinical Neurosciences Division, Charleston, SC USA
Stacia M. DeSantis, Medical University of South Carolina, Psychiatry and Behavioral Neurosciences, Clinical Neurosciences Division, Charleston, SC USA
Annie N. Simpson, Medical University of South Carolina, Psychiatry and Behavioral Neurosciences, Clinical Neurosciences Division, Charleston, SC USA
Angela E. Waldrop, Medical University of South Carolina, Psychiatry and Behavioral Neurosciences, Clinical Neurosciences Division, Charleston, SC USA
Sudie E. Back, Medical University of South Carolina, Psychiatry and Behavioral Neurosciences, Clinical Neurosciences Division, Charleston, SC USA
Mary Jeanne Kreek, The Rockefeller University
References
- 1.Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24(2):97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- 2.Koob G, Kreek MJ. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am J Psychiatry. 2007;164(8):1149–1159. doi: 10.1176/appi.ajp.2007.05030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou Y, Spangler R, Ho A, Kreek MJ. Increased CRH mRNA levels in the rat amygdala during short-term withdrawal from chronic 'binge' cocaine. Brain Res Mol Brain Res. 2003;114(1):73–79. doi: 10.1016/s0169-328x(03)00139-6. [DOI] [PubMed] [Google Scholar]
- 4.Kuhn C, Francis R. Gender difference in cocaine-induced HPA axis activation. Neuropsychopharmacology. 1997;16(6):399–407. doi: 10.1016/S0893-133X(96)00278-3. [DOI] [PubMed] [Google Scholar]
- 5.Rivier C, Vale W. Cocaine stimulates adrenocorticotropin (ACTH) secretion through a corticotropin-releasing factor (CRF)-mediated mechanism. Brain Res. 1987;422(2):403–406. doi: 10.1016/0006-8993(87)90953-x. [DOI] [PubMed] [Google Scholar]
- 6.Mendelson JH, Mello NK, Sholar MB, Siegel AJ, Mutschler N, Halpern J. Temporal concordance of cocaine effects on mood states and neuroendocrine hormones. Psychoneuroendocrinology. 2002;27(1–2):71–82. doi: 10.1016/s0306-4530(01)00036-1. [DOI] [PubMed] [Google Scholar]
- 7.Kreek MJ, Koob GF. Drug dependence: stress and dysregulation of brain reward pathways. Drug Alcohol Depend. 1998;51(1–2):23–47. doi: 10.1016/s0376-8716(98)00064-7. [DOI] [PubMed] [Google Scholar]
- 8.Goeders NE. Lapses and reinstatement of heroin seeking in rats: comment on Leri and Stewart (2002) Exp Clin Psychopharmacol. 2002;10(4):356–358. doi: 10.1037//1064-1297.10.4.356. discussion 364-356. [DOI] [PubMed] [Google Scholar]
- 9.Zhou Y, Spangler R, LaForge KS, Maggos CE, Ho A, Kreek MJ. Corticotropin-releasing factor and type 1 corticotropin-releasing factor receptor messenger RNAs in rat brain and pituitary during "binge"-pattern cocaine administration and chronic withdrawal. J Pharmacol Exp Ther. 1996;279(1):351–358. [PubMed] [Google Scholar]
- 10.Aouizerate B, Ho A, Schluger JH, Perret G, Borg L, Le Moal M, Piazza PV, Kreek MJ. Glucocorticoid negative feedback in methadone-maintained former heroin addicts with ongoing cocaine dependence: dose-response to dexamethasone suppression. Addict Biol. 2006;11(1):84–96. doi: 10.1111/j.1369-1600.2006.00006.x. [DOI] [PubMed] [Google Scholar]
- 11.Mendelson JH, Sholar M, Mello NK, Teoh SK, Sholar JW. Cocaine tolerance: behavioral, cardiovascular, and neuroendocrine function in men. Neuropsychopharmacology. 1998;18(4):263–271. doi: 10.1016/S0893-133X(97)00146-2. [DOI] [PubMed] [Google Scholar]
- 12.Herman JP, Cullinan WE. Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci. 1997;20(2):78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- 13.Shaham Y, Erb S, Stewart J. Stress-induced relapse to heroin and cocaine seeking in rats: a review. Brain Res Brain Res Rev. 2000;33(1):13–33. doi: 10.1016/s0165-0173(00)00024-2. [DOI] [PubMed] [Google Scholar]
- 14.McCance-Katz EF, Carroll KM, Rounsaville BJ. Gender differences in treatment-seeking cocaine abusers--implications for treatment and prognosis. Am J Addict. 1999;8(4):300–311. doi: 10.1080/105504999305703. [DOI] [PubMed] [Google Scholar]
- 15.Hernandez-Avila CA, Rounsaville BJ, Kranzler HR. Opioid-, cannabis-and alcohol-dependent women show more rapid progression to substance abuse treatment. Drug Alcohol Depend. 2004;74(3):265–272. doi: 10.1016/j.drugalcdep.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 16.Brady KT, Clary CM. Affective and anxiety comorbidity in post-traumatic stress disorder treatment trials of sertraline. Compr Psychiatry. 2003;44(5):360–369. doi: 10.1016/S0010-440X(03)00111-1. [DOI] [PubMed] [Google Scholar]
- 17.Kudielka BM, Kirschbaum C. Sex differences in HPA axis responses to stress: a review. Biol Psychol. 2005;69(1):113–132. doi: 10.1016/j.biopsycho.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 18.Patchev VK, Almeida OF. Gender specificity in the neural regulation of the response to stress: new leads from classical paradigms. Mol Neurobiol. 1998;16(1):63–77. doi: 10.1007/BF02740603. [DOI] [PubMed] [Google Scholar]
- 19.Handa RJ, Burgess LH, Kerr JE, O'Keefe JA. Gonadal steroid hormone receptors and sex differences in the hypothalamo-pituitary-adrenal axis. Horm Behav. 1994;28(4):464–476. doi: 10.1006/hbeh.1994.1044. [DOI] [PubMed] [Google Scholar]
- 20.Carey MP, Deterd CH, de Koning J, Helmerhorst F, de Kloet ER. The influence of ovarian steroids on hypothalamic-pituitary-adrenal regulation in the female rat. J Endocrinol. 1995;144(2):311–321. doi: 10.1677/joe.0.1440311. [DOI] [PubMed] [Google Scholar]
- 21.Windle RJ, Gamble LE, Kershaw YM, Wood SA, Lightman SL, Ingram CD. Gonadal steroid modulation of stress-induced hypothalamo-pituitary-adrenal activity and anxiety behavior: role of central oxytocin. Endocrinology. 2006;147(5):2423–2431. doi: 10.1210/en.2005-1079. [DOI] [PubMed] [Google Scholar]
- 22.First MB, Frances AJ, Pincus HA, Vettorello N, Davis WW. DSM-IV in progress. Changes in substance-related, schizophrenic, and other primarily adult disorders. Hosp Community Psychiatry. 1994;45(1):18–20. doi: 10.1176/ps.45.1.18. [DOI] [PubMed] [Google Scholar]
- 23.Sobell LC, Sobell MB. Timeline follow-back: A technique for assessing self-reported ethanol consumption. In: Allen J, Litten RZ, editors. Measuring Alcohol Consumption: Psychological And Biological Methods. Totowa, NJ: Humana Press; 1992. pp. 41–72. [Google Scholar]
- 24.Childress AR, McLellan AT, O'Brien CP. Conditioned responses in a methadone population. A comparison of laboratory, clinic, and natural settings. J Subst Abuse Treat. 1986;3(3):173–179. doi: 10.1016/0740-5472(86)90018-8. [DOI] [PubMed] [Google Scholar]
- 25.Koob GF, Heinrichs SC. A role for corticotropin releasing factor and urocortin in behavioral responses to stressors. Brain Res. 1999;848(1–2):141–152. doi: 10.1016/s0006-8993(99)01991-5. [DOI] [PubMed] [Google Scholar]
- 26.Aouizerate B, Ho A, Schluger JH, Perret G, Borg L, Le Moal M, Piazza PV, Kreek MJ. Glucocorticoid negative feedback in methadone-maintained former heroin addicts with ongoing cocaine dependence: dose-response to dexamethasone suppression. Addiction Biol. 2006;11(1):84–96. doi: 10.1111/j.1369-1600.2006.00006.x. [DOI] [PubMed] [Google Scholar]
- 27.Valentino RJ, Foote SL, Page ME. The locus coeruleus as a site for integrating corticotropin-releasing factor and noradrenergic mediation of stress responses. Ann N Y Acad Sci. 1993;697:173–188. doi: 10.1111/j.1749-6632.1993.tb49931.x. [DOI] [PubMed] [Google Scholar]
- 28.Van Bockstaele EJ, Colago EE, Valentino RJ. Amygdaloid corticotropin-releasing factor targets locus coeruleus dendrites: substrate for the co-ordination of emotional and cognitive limbs of the stress response. J Neuroendocrinol. 1998;10(10):743–757. doi: 10.1046/j.1365-2826.1998.00254.x. [DOI] [PubMed] [Google Scholar]
- 29.McDougle CJ, Black JE, Malison RT, Zimmermann RC, Kosten TR, Heninger GR, Price LH. Noradrenergic dysregulation during discontinuation of cocaine use in addicts. Arch Gen Psychiatry. 1994;51(9):713–719. doi: 10.1001/archpsyc.1994.03950090045007. [DOI] [PubMed] [Google Scholar]
- 30.Kennedy JA, Hartman N, Sbriglio R, Khuri E, Kreek MJ. Metyrapone-induced withdrawal symptoms. Br J Addict. 1990;85(9):1133–1140. doi: 10.1111/j.1360-0443.1990.tb03438.x. [DOI] [PubMed] [Google Scholar]
- 31.Sinha R, Talih M, Malison R, Cooney N, Anderson GM, Kreek MJ. Hypothalamic-pituitary-adrenal axis and sympatho-adreno-medullary responses during stress-induced and drug cue-induced cocaine craving states. Psychopharmacology. 2003;170(1):62–72. doi: 10.1007/s00213-003-1525-8. [DOI] [PubMed] [Google Scholar]
- 32.Sinha R, Li CS. Imaging stress-and cue-induced drug and alcohol craving: association with relapse and clinical implications. Drug Alcohol Rev. 2007;26(1):25–31. doi: 10.1080/09595230601036960. [DOI] [PubMed] [Google Scholar]
- 33.Sinha R, Garcia M, Paliwal P, Kreek MJ, Rounsaville BJ. Stress-induced cocaine craving and hypothalamic-pituitary-adrenal responses are predictive of cocaine relapse outcomes. Arch Gen Psychiatry. 2006;63(3):324–331. doi: 10.1001/archpsyc.63.3.324. [DOI] [PubMed] [Google Scholar]
- 34.Brady KT, Back SE, Waldrop AE, McRae AL, Anton RF, Upadhyaya HP, Saladin ME, Randall PK. Cold pressor task reactivity: predictors of alcohol use among alcohol-dependent individuals with and without comorbid posttraumatic stress disorder. Alcohol Clinical Exp Res. 2006;30(6):938–946. doi: 10.1111/j.1530-0277.2006.00097.x. [DOI] [PubMed] [Google Scholar]
- 35.Junghanns K, Backhaus J, Tietz U, Lange W, Bernzen J, Wetterling T, Rink L, Driessen M. Impaired serum cortisol stress response is a predictor of early relapse. Alcohol Alcohol. 2003;38(2):189–193. doi: 10.1093/alcalc/agg052. [DOI] [PubMed] [Google Scholar]
- 36.Contoreggi C, Herning RI, Na P, Gold PW, Chrousos G, Negro PJ, Better W, Cadet JL. Stress hormone responses to corticotropin-releasing hormone in substance abusers without severe comorbid psychiatric disease. Biol Psychiatry. 2003;54(9):873–878. doi: 10.1016/s0006-3223(03)00167-7. [DOI] [PubMed] [Google Scholar]
- 37.Adinoff B, Krebaum SR, Chandler PA, Ye W, Brown MB, Williams MJ. Dissection of Hypothalamic-Pituitary-Adrenal Axis Pathology in 1-Month-Abstinent Alcohol-Dependent Men, Part 2: Response to Ovine Corticotropin-Releasing Factor and Naloxone. Alcohol Clinical Exp Res. 2005;29(4):528–537. doi: 10.1097/01.ALC.0000158939.25531.EE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mendelson JH, Teoh SK, Lange U, Mello NK, Weiss R, Skupny A, Ellingboe J. Anterior pituitary, adrenal, and gonadal hormones during cocaine withdrawal. Am J Psychiatry. 1988;145(9):1094–1098. doi: 10.1176/ajp.145.9.1094. [DOI] [PubMed] [Google Scholar]
- 39.Mendelson JH, Mello NK, Teoh SK, Ellingboe J, Cochin J. Cocaine effects on pulsatile secretion of anterior pituitary, gonadal, and adrenal hormones. J Clin Endocrinol Metab. 1989;69(6):1256–1260. doi: 10.1210/jcem-69-6-1256. [DOI] [PubMed] [Google Scholar]
- 40.Schluger JH, Bart G, Green M, Ho A, Kreek MJ. Corticotropin-releasing factor testing reveals a dose-dependent difference in methadone maintained vs control subjects. Neuropsychopharmacology. 2003;28(5):985–994. doi: 10.1038/sj.npp.1300156. [DOI] [PubMed] [Google Scholar]
- 41.Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, Hellhammer DH. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosom Med. 1999;61(2):154–162. doi: 10.1097/00006842-199903000-00006. [DOI] [PubMed] [Google Scholar]
- 42.Kudielka BM, Kirschbaum C. Sex differences in HPA axis responses to stress: a review. Biol Psychol. 2005;69(1):113–132. doi: 10.1016/j.biopsycho.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 43.Gianoulakis C, Dai X, Brown T. Effect of chronic alcohol consumption on the activity of the hypothalamic-pituitary-adrenal axis and pituitary beta-endorphin as a function of alcohol intake, age, and gender. Alcohol Clin Exp Res. 2003;27(3):410–423. doi: 10.1097/01.ALC.0000056614.96137.B8. [DOI] [PubMed] [Google Scholar]
- 44.Back SE, Waldrop AE, Saladin ME, Yeatts SD, Simpson A, McRae AL, Upadhyaya HP, Contini Sisson R, Spratt EG, Allen J, Kreek MJ, Brady KT. Effects of gender and cigarette smoking on reactivity to psychological and pharmacological stress provocation. Psychoneuroendocrinology. 2008 doi: 10.1016/j.psyneuen.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chatham LR, Hiller ML, Rowan-Szal GA, Joe GW, Simpson DD. Gender differences at admission and follow-up in a sample of methadone maintenance clients. Subst Use Misuse. 1999;34(8):1137–1165. doi: 10.3109/10826089909039401. [DOI] [PubMed] [Google Scholar]
- 46.Gentilello LM, Rivara FP, Donovan DM, Villaveces A, Daranciang E, Dunn CW, Ries RR. Alcohol problems in women admitted to a level I trauma center: a gender-based comparison. J Trauma. 2000;48(1):108–114. doi: 10.1097/00005373-200001000-00018. [DOI] [PubMed] [Google Scholar]
- 47.Henskens R, Mulder CL, Garretsen H. Gender differences in problems and needs among chronic, high-risk crack abusers: Results of a randomized controlled trial. J Subst Abuse Treat. 2005;10:128–140. [Google Scholar]
- 48.Mann K, Ackermann K, Croissant B, Mundle G, Nakovics H, Diehl A. Neuroimaging of gender differences in alcohol dependence: are women more vulnerable? Alcohol Clinical Exp Res. 2005;29(5):896–901. doi: 10.1097/01.alc.0000164376.69978.6b. [DOI] [PubMed] [Google Scholar]
- 49.Johnson EO, Schultz L. Forward telescoping bias in reported age of onset: an example from cigarette smoking. Int J Methods Psychiatr Res. 2005;14(3):119–129. doi: 10.1002/mpr.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Randall CL, Roberts JS, Del Boca FK, Carroll KM, Connors GJ, Mattson ME. Telescoping of landmark events associated with drinking: a gender comparison. J Stud Alcohol. 1999;60(2):252–260. doi: 10.15288/jsa.1999.60.252. [DOI] [PubMed] [Google Scholar]