Abstract
Chromosome translocations in peripheral blood lymphocytes of normal, healthy humans increase with age, but the effects of gender, race, and cigarette smoking on background translocation yields have not been examined systematically. Further, the shape of the relationship between age and translocation frequency (TF) has not been definitively determined. We collected existing data from sixteen laboratories in North America, Europe, and Asia on TFs measured in peripheral blood lymphocytes by fluorescence in situ hybridization whole chromosome painting among 1933 individuals. In Poisson regression models, age, ranging from newborns (cord blood) to 85 years, was strongly associated with TF and this relationship showed significant upward curvature at older ages vs. a linear relationship (p <0.001). Ever smokers had significantly higher TFs than non-smokers (rate ratio (RR) = 1.19, 95% confidence interval (CI), 1.09–1.30) and smoking modified the effect of age on TFs with a steeper age-related increase among ever smokers compared to non-smokers (p<0.001). TFs did not differ by gender. Interpreting an independent effect of race was difficult owing to laboratory variation. Our study is three times larger than any pooled effort to date, confirming a suspected curvilinear relationship of TF with age. The significant effect of cigarette smoking has not been observed with previous pooled studies of TF in humans. Our data provide stable estimates of background TF by age, gender, race, and smoking status and suggest an acceleration of chromosome damage above age 60 and among those with a history of smoking cigarettes.
Keywords: chromosome translocations, background frequency, controls, fluorescence in situ hybridization
1. Introduction
Chromosome aberrations are a biological marker of clastogenic exposures and of cancer susceptibility [1–4]. Genotoxic agents, including chemicals and ionizing radiation, induce chromosomal aberrations including translocations. Because translocations frequently persist through mitosis so that the affected cells and their progeny remain viable, they are of interest as a biomarker because they allow for meaningful assessment many years after the putative exposure has occurred [5–9]. Increased frequencies of stable and unstable chromosome aberrations are known to occur after radiation exposure and to increase with age. However the effects of gender, ethnicity, and lifestyle factors (such as cigarette smoking) on background translocation yields have been difficult to assess due to small sample sizes [10–15]. For many years chromosome aberration frequencies have been known to increase with age [16–20], but the relationship between translocation frequencies and age has not been well characterized. More precise estimates of background translocation levels in humans are needed [21,22]. Stable baseline estimates will be very valuable for providing a comparison group to study populations that have been chronically exposed to environmental genotoxins at very low levels. In such cases it would be difficult to detect differences unless the exposed group was large and many thousands of cells are scored [9]. Pooled analyses of translocation data in unexposed individuals using fluorescence in situ hybridization (FISH) whole chromosome painting have been conducted among 436 and 385 persons [23,24]. In these and other studies, background translocation frequencies were strongly influenced by age with a suggestion of an upward curvature at older ages [10–12,23–25]. However, whether the translocation and age relationship was modified by gender, race, or smoking has remained unclear. Here we extend these earlier efforts by expanding the number of international laboratories contributing data using FISH painting to determine the effects of age, gender, ethnicity, and cigarette smoking on baseline translocation frequencies.
2. Methods
2.1 Study design and data collection
Potential contributing laboratories with FISH chromosome painting translocation data were identified from knowledge of several co-authors and from PubMed searches using the MESHterms “radiation dose, human, FISH, cytogenetics, chromosome aberrations”. Laboratories and individual investigators were contacted and asked to provide data on the subjects in their previous or ongoing studies who were unexposed to ionizing radiation other than radiation from natural background sources or routine personal medical diagnostic procedures. Each laboratory sent data on individuals without personal identifying information. This study was based on anonymized data, for which a National Institutes of Health human subjects exemption was obtained from the Office of Human Subjects Research.
A pooled analysis of data from peripheral blood lymphocytes was performed on 1933 persons from 16 laboratories who were studied either to determine background translocation frequencies or had served as controls in studies of radiation- or chemical-exposed populations. We requested information on age at time of blood collection, gender, ethnicity, and cigarette smoking status. Newborns, from whom cord blood was obtained, were assigned as cigarette smokers or non-smokers based on the smoking status of the mother during pregnancy. For all subjects we collected data on the specific chromosomes painted, number of paint colors used, number of metaphase cells scored, and the number of translocations counted. Translocations, whether apparently reciprocal (“two-way”) or non-reciprocal (“one-way”), were counted as a single translocation event. The premise for the single count was that most non-reciprocal translocations are in fact reciprocal at the molecular level [26] and that under these conditions the frequency of complex rearrangements is negligible.
2.2 Blood sample collection and culture methods
All of the blood was collected following informed consent and the blood drawing had been approved by each laboratory’s human subjects review board. Individual laboratories cultured the blood samples and painted various combinations of chromosomes according to their routine practices [10,15,27–35]. The specific chromosomes that were painted and the number of colors used by each laboratory are shown in Table 1. Lymphocyte cultures were incubated typically for 48 hours, which is known to produce cells that are primarily in their first mitotic division in vitro. Some cells were cultured for as long as 72 hours. Although these cultures will contain many cells in their second division, this should not have a large effect on the translocation yield because translocation frequencies in cultured human peripheral blood lymphocytes do not show major changes during the first 72 hours [33].
Table 1.
Contributing laboratories and descriptive features of chromosome painting techniques.
| Country | Laboratory* | Chromosomes painted† | Number of colors with no counterstain | Fraction of exchanges detected‡ | Average number of cell equivalents | Range of cell equivalents |
|---|---|---|---|---|---|---|
| Germany | BfS | 2,4,8 | 1 | 0.303 | 773 | 608–1184 |
| Canada | Health and Welfare | 2,3,4 | 3 | 0.353 | 420 | 346–726 |
| Russia | CRIRR | 1,4,12 (primarily) | 1 | 0.302 | 385 | 147–1042 |
| Germany | GSF | 1,4,12 | 1 | 0.302 | 548 | 188–906 |
| United Kingdom | HPA | 2,3,5 | 1 | 0.322 | 935 | 629–1288 |
| France | IRSN | 2,4,12 | 3 | 0.321 | 479 | 167–1072 |
| Czech Republic | LGE | 1,4 | 2 | 0.253 | 253 | 246–321 |
| United States | LLNL (n=282) | 1,2,4† | 1 | 0.343 | 826 | 216–1944 |
| United States | LLNL (n=512) | 1,2,4 & 3,5,6 | 2 | 0.559 | 996 | 203–2259 |
| The Netherlands | LUMC | 1,4,8 | 3 | 0.33 | 433 | 164–662 |
| United States | ORAU | 1,2,4 | 1 | 0.343 | 544 | 380–1279 |
| Japan and China | NIRS & NIRP & CCDCP | 1,2,4 | 1 | 0.343 | 1536 | 224–7437 |
| Japan | RERF | 1,2,4 | 1 | 0.343 | 172 | 144–206 |
| Korea | SNU | 1,4 | 2 | 0.253 | 127 | 127–127 |
| Finland | STUK | 1,2,4 | 1 | 0.343 | 642 | 502–1199 |
| Spain | UAB | 1,4,11 | 1 | 0.302 | 444 | 358–530 |
| United Kingdom | WRI | 1,3,4 (primarily) | 1 | 0.328 | 386 | 321–656 |
BfS: Federal Office for Radiation Protection; CRIRR: Central Research Institute of Roentgenology and Radiology; GSF: National Research Center for Environment and Health, Institute of Radiobiology; HPA: Health Protection Agency, Radiation Protection Division (formerly the National Radiological Protection Board (NRPB)); IRSN: Institut de Radioprotection et Sûreté Nucléaire; LGE: Laboratory of Genetic Ecotoxicology; LLNL: Lawrence Livermore National Laboratory; LUMC: Leiden University Medical Centre; NIRS: National Institute of Radiological Sciences; NIRP, CCDCP: National Institute for Radiological Protection, Chinese Center for Disease Control and Prevention; ORAU: Oak Ridge Associated Universities; RERF: Radiation Effects Research Foundation; SNU: Seoul National University; STUK: Radiation and Nuclear Safety Authority; UAB: Universitat Autonoma de Barcelona; WRI: Westlakes Research Institute.
Some laboratories reported painting other chromosomes or varying the number of colors: CRIRR had 7 subjects (16%), LLNL had 14 subjects (5%), and WRI had 23 subjects (17%) for which the chromosome painting descriptions differed slightly from the table. These differences were included in the calculations for the aggregated data.
The fraction of exchanges detected was calculated using equations appropriate for one- to three-color paints [31] and was based on the DNA content of the painted chromosomes [32]. The proportion of genome coverage differs slightly for males and females due to the larger size of the X compared to the Y chromosome and some laboratories adjust for these gender differences in their calculations. For ease of presentation we show here the proportion for men, which is minimally larger than for women, however the calculations for the aggregated data were performed using gender-specific equations as described in the text.
2.3 Scoring methodology
Well-spread metaphase cells were considered suitable for scoring if the cells appeared to be intact, the centromeres were morphologically detectable and present in all the painted chromosomes, and the fluorochrome labeling was sufficiently bright to detect exchanges between chromosomes labeled in different colors. See Figure 1 for a photograph of exchanges occurring between painted chromosomes. Clonal cells were identified in some samples from some laboratories. Where present, clones were defined as three or more cells with the same translocation, and translocations in clonal cells were counted only once. For some of the laboratories located in Western Europe, only stable cells were eligible for inclusion. Stable cells were defined as those not containing any dicentric, centric ring, or acentric fragment in either the stained or counter-stained chromosomes. Because unstable cells are rare among persons having negligible exposure to radiation, they may be practically ignored as only trivially contributing to between-laboratory differences [24]. Conversion of metaphase cells scored to whole-genome equivalents (defined as cell equivalents or CEs) was calculated using equations appropriate for one-color to three-color paints [36]. Due to small variations in the methods that each contributing laboratory used to calculate CEs, we re-calculated them in a standardized manner based on the DNA content of the painted chromosomes [37], including the X and Y chromosomes according to the gender of each donor.
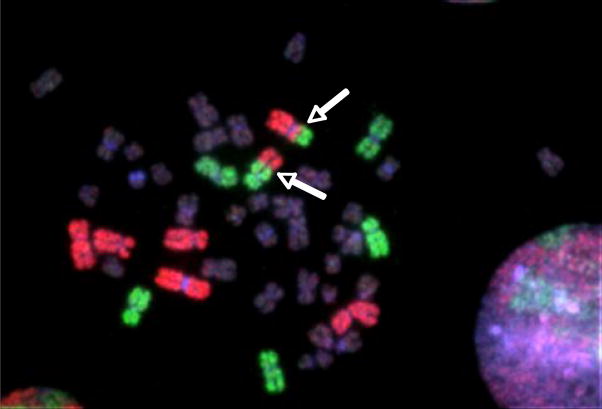
Figure 1.
Human cell with an apparently reciprocal chromosome translocation (arrows) detected by fluorescence in situ hybridization (FISH) using whole chromosome paints. Chromosome pairs 1, 2, and 4 are painted red, and 3, 5, and 6 are painted green.
2.4 Data inclusion criteria
The data for individual subjects were considered eligible for inclusion if they met the following criteria: complete information on gender, race, age, and smoking status; 100 or more CEs evaluated; and no exposure to ionizing radiation except for natural background levels or for routine medical diagnostic procedures. In total there were data available for 2067 individuals. We excluded 127 subjects due to missing data on age, gender, race or smoking; four subjects were eliminated because they had fewer than 100 CEs evaluated; and three subjects were removed because they had undergone prior therapeutic radiation, leaving a total of 1933 subjects who were evaluated. Of these, 1400 subjects had at least 300 CEs scored, and 533 subjects had more than 100 CEs but fewer than 300 CEs evaluated.
2.5 Statistical methods
For each individual, we calculated the translocation frequency (TF) as the number of translocations per 100 CE. While called “frequency”, for statistical purposes this should more appropriately be thought of as a rate, hence comparisons between groups are rate ratios. Means and standard deviations of TFs were calculated within 5-year age intervals and by categories of other covariates. Poisson regression was used to evaluate the association between TFs and the covariates of age, smoking cigarettes (ever vs. never), gender, race (Asian, Black, White, Other), and laboratory, by estimating rate ratios for categories of one covariate relative to a reference category adjusted for all other covariates. A Pearson scale factor was used to correct for over-dispersion. All calculations were performed with EPICURE software [38].
The shape of the relationship between TF and age was evaluated based on a linear-exponential model
where the intercept α represents the translocation rate at age 0 (i.e., birth), the linear slope parameter β represents the increase in translocation rate per year of age, and the loglinear curvature parameter γ measures downward concavity (γ <0) or upward convexity (γ >0) with age, and thus the degree of departure from linearity. A test of the null hypothesis, γ=0, is a test of no departure from a linear relationship in age. For convenience in fitting this model, exp(α*) and exp(β*) replace α and β, respectively, to avoid range restrictions on the parameters. We considered other ways to model non-linearity, including fully loglinear models with and without a quadratic term for age. Models were compared by visual inspection of the corresponding plots and by assessing the goodness of fit based on the deviance, even though not all of these models are nested and formal statistical comparisons based on the deviance were therefore not possible.
We evaluated potential modifications of the relationship between TF and age by smoking, gender, race, and laboratory using likelihood ratio tests to assess whether the slope or curvature, or both, varied across categories of the potential effect modifier. To assess the main effect of laboratory and its potential role as a modifier of the relationship between TF and age, we collapsed the 16 laboratories into four geographic regions: Asia included laboratories located in Japan and Korea; Central and Eastern Europe included laboratories in the Czech Republic and Russia; North America included laboratories in the United States and Canada; Western Europe included laboratories in the United Kingdom, France, Spain, Germany, Finland, and the Netherlands.
We evaluated the consistency of our results by using several approaches. We stratified by geographic region and also further restricted our analyses to individuals in whom 300 CEs or more scored. We ultimately relied on data based on at least 300 CEs to reduce the inter-laboratory variability in TF scoring.
3. Results
Table 1 displays the descriptive features of the chromosome painting and average number of cell equivalents analyzed by each laboratory. A total of 1933 persons were included, who ranged in age from zero (cord blood from newborns) to 85 years with a mean age of 43 years of whom 39% were women, 9% Asian, 13% Black, 75% White, and 2% were other races (Table 2). Thirty-nine percent reported ever (current or former) cigarette smoking. Means of the translocation frequencies by age for all the study subjects combined and by gender are shown in Table 3. These unadjusted translocation frequencies were slightly lower in women than men up to about age 50, and then became higher among women than men. The rate ratios, which were restricted to those for whom at least 300 CEs were scored and were based on a multivariate model including all covariates, increased with age, and were marginally lower in women compared to men. Rate ratios were significantly higher among cigarette smokers compared to non-smokers (rate ratio (RR) = 1.19; 95% confidence interval (CI), 1.09–1.30) (Table 4). The rate ratios for Asian and those of other races were not significantly different from Whites, however Blacks had lower TFs compared to Whites (RR=0.84; 95% CI, 0.71–0.99). TFs from laboratories in Asia and Western Europe were not statistically significantly different from North American laboratories, but Central and Eastern European laboratories were associated with higher TFs (RR=1.75; 95% CI, 1.24–2.47) compared to the other laboratories.
Table 2.
Demographic characteristics and cigarette smoking status of all individuals by laboratory from whom at least 100 CEs were scored.
| Gender | Age in years† | Race | Cigarette smoking | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Laboratory* | Number of individuals | Male | Female | Mean | min | max | Asian | Black | White | Never | Ever |
| BfS | 53 | 33 | 20 | 48 | 21 | 85 | 0 | 0 | 53 | 18 | 35 |
| Health and Welfare Canada | 15 | 8 | 7 | 41 | 27 | 65 | 0 | 0 | 15 | 4 | 11 |
| CRIRR | 45 | 26 | 19 | 29 | 3 | 81 | 0 | 0 | 45 | 32 | 13 |
| GSF | 53 | 36 | 17 | 47 | 9 | 84 | 0 | 0 | 53 | 47 | 6 |
| HPA | 47 | 34 | 13 | 40 | 20 | 64 | 1 | 20 | 26 | 36 | 11 |
| IRSN | 35 | 20 | 15 | 39 | 20 | 67 | 0 | 0 | 35 | 21 | 14 |
| LGE | 346 | 275 | 71 | 34 | 6 | 65 | 0 | 0 | 346 | 263 | 83 |
| LLNL‡ | 794 | 378 | 416 | 22 | 0 | 79 | 9 | 237 | 509 | 437 | 357 |
| LUMC | 35 | 18 | 17 | 45 | 24 | 70 | 0 | 0 | 35 | 29 | 6 |
| NIRS and NIRP | 32 | 16 | 16 | 51 | 10 | 71 | 32 | 0 | 0 | 24 | 8 |
| ORAU | 52 | 49 | 3 | 42 | 24 | 55 | 0 | 1 | 51 | 26 | 26 |
| RERF | 113 | 44 | 69 | 67 | 50 | 85 | 113 | 0 | 0 | 71 | 42 |
| SNU | 27 | 27 | 0 | 36 | 20 | 55 | 27 | 0 | 0 | 10 | 17 |
| STUK | 127 | 79 | 48 | 49 | 17 | 72 | 0 | 0 | 127 | 95 | 32 |
| UAB | 21 | 11 | 10 | 38 | 23 | 61 | 0 | 0 | 21 | 13 | 8 |
| WRI | 138 | 132 | 6 | 56 | 0 | 84 | 0 | 0 | 138 | 52 | 86 |
| TOTAL | 1933 | 1186 | 747 | 43 | 0 | 85 | 182 | 258 | 1454 | 1178 | 755 |
See footnote in Table 1 for laboratory name and country.
min, minimum; max, maximum
The numbers for race do not sum to the total due to 39 individuals with race designated as “other”.
Table 3.
Mean translocation frequencies and 95% confidence intervals* (CI) per 100 cell equivalents by age and gender for all individuals from whom at least 100 CEs were scored.
| All |
Males |
Females |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 95% CI | 95% CI | 95% CI | ||||||||||
| Age group (years) | No. of persons | Mean | Lower | Upper | No. of men | Mean | Lower | Upper | No. of women | Mean | Lower | Upper |
| 0 | 296 | 0.04 | 0.03 | 0.05 | 149 | 0.04 | 0.03 | 0.06 | 147 | 0.03 | 0.02 | 0.05 |
| 1–4 | 3 | 0.22 | 0.03 | 1.99 | 1 | 0.33 | 0.02 | 7.36 | 2 | 0.17 | 0.01 | 2.87 |
| 5–9 | 38 | 0.15 | 0.07 | 0.32 | 19 | 0.11 | 0.03 | 0.39 | 19 | 0.19 | 0.08 | 0.46 |
| 10–14 | 29 | 0.29 | 0.21 | 0.42 | 20 | 0.22 | 0.14 | 0.36 | 9 | 0.49 | 0.31 | 0.80 |
| 15–19 | 65 | 0.20 | 0.15 | 0.27 | 17 | 0.20 | 0.09 | 0.43 | 48 | 0.20 | 0.15 | 0.27 |
| 20–24 | 191 | 0.24 | 0.21 | 0.29 | 76 | 0.38 | 0.29 | 0.50 | 115 | 0.20 | 0.16 | 0.24 |
| 25–29 | 177 | 0.42 | 0.36 | 0.48 | 95 | 0.60 | 0.49 | 0.73 | 82 | 0.30 | 0.25 | 0.37 |
| 30–34 | 138 | 0.41 | 0.34 | 0.48 | 81 | 0.53 | 0.42 | 0.66 | 57 | 0.30 | 0.24 | 0.39 |
| 35–39 | 154 | 0.52 | 0.46 | 0.60 | 107 | 0.65 | 0.56 | 0.77 | 47 | 0.31 | 0.23 | 0.40 |
| 40–44 | 141 | 0.67 | 0.59 | 0.77 | 106 | 0.67 | 0.57 | 0.78 | 35 | 0.70 | 0.53 | 0.91 |
| 45–49 | 152 | 0.91 | 0.81 | 1.02 | 124 | 0.92 | 0.81 | 1.04 | 28 | 0.87 | 0.68 | 1.11 |
| 50–54 | 140 | 0.86 | 0.76 | 0.96 | 121 | 0.83 | 0.73 | 0.94 | 19 | 1.06 | 0.81 | 1.38 |
| 55–59 | 112 | 0.91 | 0.80 | 1.04 | 82 | 0.93 | 0.80 | 1.08 | 30 | 0.87 | 0.69 | 1.09 |
| 60–64 | 87 | 1.19 | 1.04 | 1.35 | 58 | 1.14 | 0.97 | 1.33 | 29 | 1.31 | 1.07 | 1.61 |
| 65–69 | 102 | 1.22 | 1.06 | 1.40 | 62 | 1.16 | 0.95 | 1.41 | 40 | 1.29 | 1.07 | 1.56 |
| 70–74 | 61 | 1.50 | 1.31 | 1.72 | 36 | 1.49 | 1.23 | 1.80 | 25 | 1.52 | 1.26 | 1.82 |
| 75–79 | 22 | 1.71 | 1.36 | 2.14 | 14 | 1.60 | 1.21 | 2.10 | 8 | 1.99 | 1.38 | 2.87 |
| 80+ | 25 | 1.81 | 1.39 | 2.36 | 18 | 1.65 | 1.21 | 2.24 | 7 | 2.66 | 1.61 | 4.38 |
| Total | 1933 | 1186 | 747 | |||||||||
The confidence intervals were based on the asymptotic normal distribution of the estimated Poisson regression coefficient representing the expected age-specific log translocation frequency. The Poisson model also included a scale factor to account for overdispersion. The scale factor was estimated as the overall Pearson chi-square divided by its degrees of freedom.
Table 4.
Multivariate adjusted rate ratios by descriptive characteristics for individuals with 300 or more cell equivalents scoreda.
| Descriptive characteristics | Number of subjects (N = 1400) | Average number of cell equivalents | Number of translocations | Rate ratio | 95% CI | |
|---|---|---|---|---|---|---|
| Age | 0 | 294 | 985 | 103 | 0.06 | (0.04 0.09) |
| 1~4 | 2 | 373 | 2 | 0.27 | (0.03 2.36) | |
| 5~9 | 7 | 424 | 3 | 0.11 | (0.02 0.66) | |
| 10~14 | 20 | 1229 | 75 | 0.47 | (0.31 0.70) | |
| 15~19 | 60 | 854 | 99 | 0.35 | (0.24 0.49) | |
| 20~24 | 148 | 834 | 265 | 0.37 | (0.29 0.48) | |
| 25~29 | 119 | 816 | 361 | 0.64 | (0.52 0.80) | |
| 30~34 | 81 | 815 | 229 | 0.57 | (0.45 0.74) | |
| 35~39 | 106 | 761 | 375 | 0.77 | (0.62 0.96) | |
| 40~44 | 93 | 730 | 425 | 1.00 | (referent) | |
| 45~49 | 99 | 694 | 533 | 1.27 | (1.05 1.55) | |
| 50~54 | 96 | 745 | 542 | 1.25 | (1.03 1.52) | |
| 55~59 | 86 | 686 | 501 | 1.37 | (1.12 1.67) | |
| 60~64 | 61 | 726 | 488 | 1.78 | (1.45 2.18) | |
| 65~69 | 53 | 578 | 300 | 1.60 | (1.27 2.03) | |
| 70~74 | 48 | 650 | 446 | 2.29 | (1.85 2.82) | |
| 75~79 | 15 | 651 | 154 | 2.52 | (1.91 3.35) | |
| 80+ | 12 | 435 | 72 | 2.03 | (1.38 3.00) | |
| Gender | Male | 811 | 737 | 3298 | 1.00 | (referent) |
| Female | 589 | 895 | 1675 | 0.92 | (0.83 1.03) | |
| Smoking | Nonsmokers | 804 | 813 | 2610 | 1.00 | (referent) |
| Smokers | 596 | 791 | 2363 | 1.19 | (1.09 1.30) | |
| Race | White | 1064 | 730 | 4061 | 1.00 | (referent) |
| Asian | 41 | 1027 | 452 | 1.23 | (0.63 2.38) | |
| Black | 256 | 1445 | 392 | 0.84 | (0.71 0.99) | |
| Others | 39 | 673 | 68 | 1.06 | (0.72 1.57) | |
| Laboratory regionb | North America | 856 | 908 | 2438 | 1.00 | (referent) |
| Asia | 31 | 1578 | 371 | 0.86 | (0.43 1.72) | |
| Central and Eastern Europe | 29 | 476 | 86 | 1.75 | (1.24 2.47) | |
| Western Europe | 484 | 589 | 2078 | 0.99 | (0.89 1.09) | |
To calculate the absolute expected translocation rate for a given combination of values of the covariates shown, multiply the estimated model intercept, i.e., 0.59, by the relative effects shown in the table, e.g., for a 62-year old Black male smoker who was evaluated in a North American laboratory, the expected translocation rate is calculated as 0.59 × 1.78 × 1.00 × 1.19 × 0.84 × 1.00=1.04 translocations per 100 cells. The equation does not account for any effect modification by cigarette smoking or race.
Asia: NIRS and NIRP, RERF, SNU; Central and Eastern Europe: CRIRR, LGE; North America: Health and Welfare Canada, LLNL, ORAU; Western Europe: BfS, GSF, HPA, IRSN, LUMC, STUK, UAB, WRI
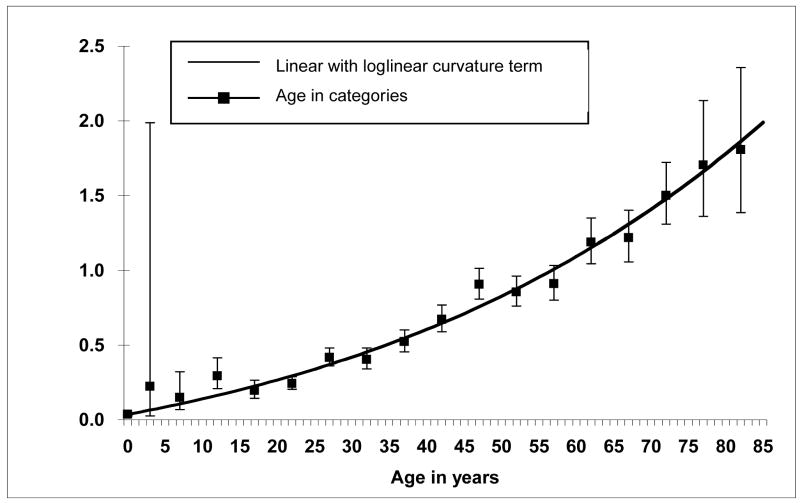
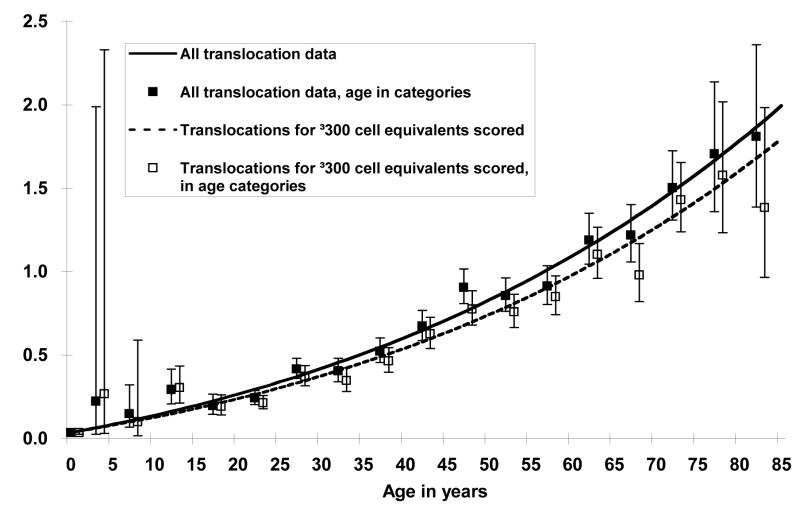
The slope and curvature terms of a model for age were estimated to be larger than zero and were highly statistically significant (p <0.001 for both). The model fit the data well (Figure 2) and resulted in a lower deviance (4186.6) compared to a loglinear model with a quadratic term (4235.8) with a similar number of parameters, although the models are not nested and formal statistical comparisons based on the deviance are therefore not possible. These results indicate that the TF increases with increasing age and that upward curvature occurs for ages above 60 years. Excluding individuals from whom fewer than 300 CEs were scored resulted in slightly lower age-specific mean TFs with the difference between the curves becoming more pronounced at older ages, but a linear model with loglinear curvature term resulted in highly significant parameter estimates (p<0.001 for both) and continued to describe the data best (Figure 3).
Figure 2.
Translocation frequencies per 100 cell equivalents by age, with means and 95% confidence bounds for age in 5-year categories for all 1933 subjects. The equation for the line is TR(age)=100(exp(−7.925)+exp(−9.284)*(age*exp0.01062*age)) where TR is the translocation rate and age is in years.
Figure 3.
Age-specific translocation frequencies using all the data (N=1933) compared to the plot using data restricted to individuals for whom at least 300 cell equivalents (CE) were evaluated (N=1400). Age-specific mean translocation frequencies were slightly lower when the data were limited to 300 CEs or greater, however a linear model with a loglinear curvature term continued to fit the data best.
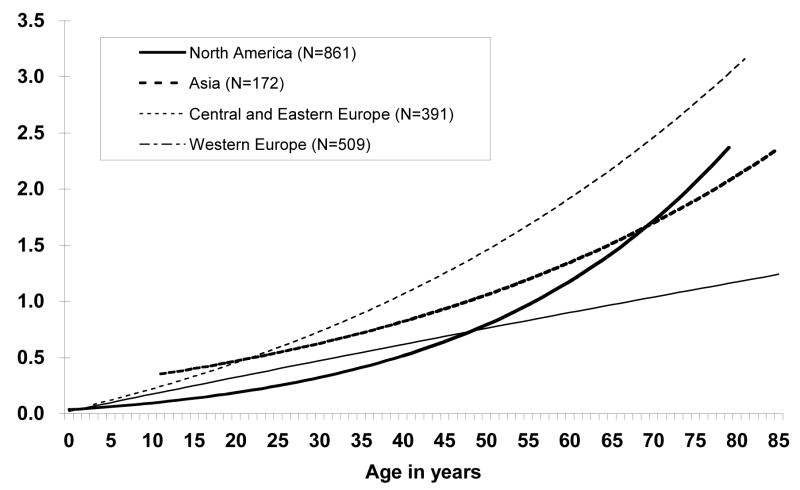
Age-related TFs differed significantly between geographic region (p<0.001), and the age-specific TFs were best described with a linear slope and loglinear curvature term for Central and Eastern European and North American laboratories with all slope and curvature parameters significantly larger than zero (p<0.01 and p<0.01 for Central and Eastern Europe, p<0.01 and p<0.001 for North America, respectively). In contrast, the curvature term for Western European laboratories was not significantly different from zero (p=0.40) so that the relationship between TF and age was best described with a linear slope term alone (Figure 4). Age-related TFs were best described with a log-linear model for the Asian laboratories. Age-specific translocation frequencies under 60 years were highest in Central and Eastern European laboratories, intermediate in the Asian laboratories, and similar for North American and Western European laboratories. For individuals over age 60 years, the age-specific translocation frequencies remained highest in the Central and Eastern European laboratories, intermediate in the Asian and North American laboratories, and lowest in the Western European laboratories.
Figure 4.
Translocation frequencies per 100 cell equivalents by age and geographic region of laboratory. The Geographic laboratory region significantly altered the age and translocation frequency relationship (p < 0.001). Laboratories associated with their respective geographic regions were: Asia: NIRS, RERF, SNU; Central and Eastern Europe: CRIRR, LGE; North America: Health and Welfare Canada, LLNL, ORAU; Western Europe: BfS, GSF, HPA, IRSN, LUMC, STUK, UAB, WRI. Number of control individuals for each laboratory region are in parentheses.
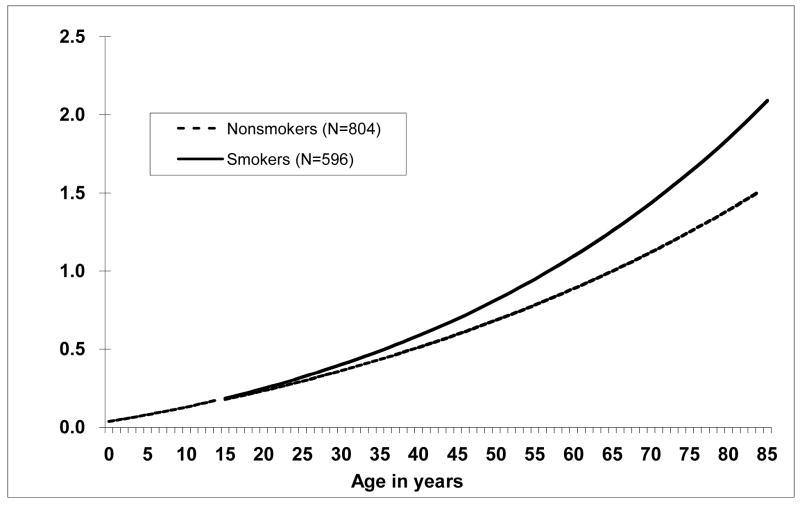
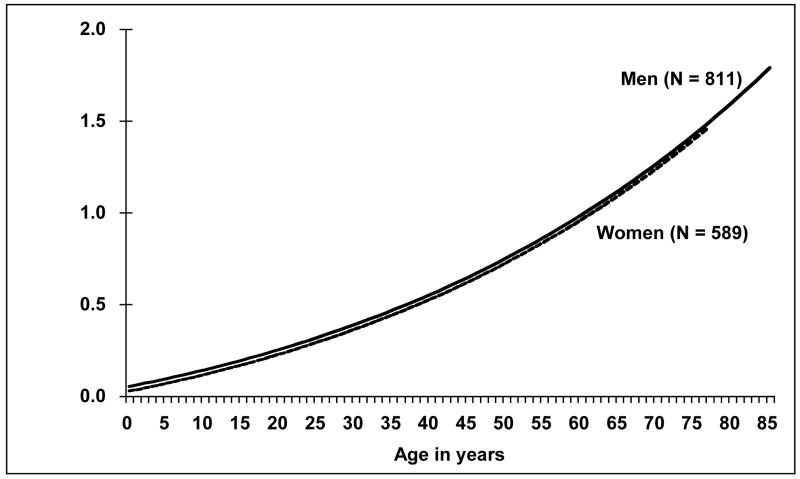
Smoking significantly modified the TF and age relationship (p < 0.001) when the data were restricted to individuals with at least 300 CEs scored, with ever smokers having steeper age-specific TFs than non-smokers (Figure 5). Gender did not significantly influence the age-specific translocation frequencies when the data were restricted to those with at least 300 CEs scored (p = 0.10, Figure 6).
Figure 5.
Translocation frequencies per 100 cell equivalents by age and smoking status. Ever having smoked cigarettes significantly altered the age and translocation frequency relationship, p < 0.001. Number of individuals appears in parentheses.
Figure 6.
Age-specific translocation frequencies by gender. Gender did not significantly modify the age and translocation frequency relationship, p = 0.09. Number of individuals appears in parentheses.
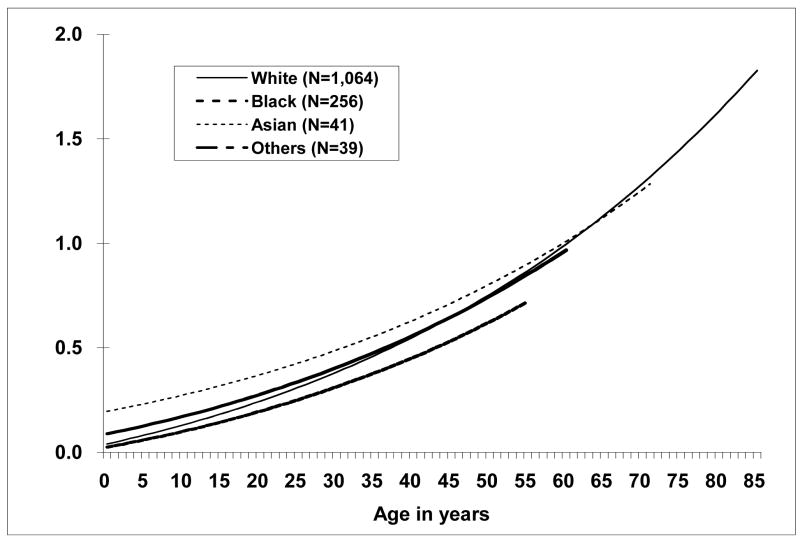
Race significantly altered the relationship between TF and age (p=0.002) when at least 300 CEs were scored (Figure 7). Asians tended to have higher TFs than Blacks, with the TFs for Whites being more similar to Blacks at young ages (< 50 years) and more similar to Asians at older ages (> 50 years).
Figure 7.
Age-specific translocation frequencies by race. Race significantly modified the age and translocation frequency relationship, p = 0.003. Number of individuals appears in parentheses.
4. Discussion
We have shown that chromosome translocation frequencies (expressed per 100 CEs) in peripheral blood lymphocytes of normal, healthy people increase with increasing age. The data indicated an upwardly curving relationship, i.e. the rate of increase is also increasing with age. We further evaluated the consistency of this observation by restricting the data analyzed to the scoring of at least 300 cell equivalents per subject, which reduced the variability. The shape of the relationship remained unchanged, supporting our observation that at older ages there was an apparent acceleration in chromosome damage after about age 60. Ever smoking cigarettes appeared to be related to higher TFs than for never smokers. Smoking further modified the TF and age relationship with a steeper increase in smokers than non-smokers. Female gender was associated with a non-significantly lower TF but gender did not alter the TF and age relationship when accounting for the effect of smoking, race, and laboratory. We found significant differences in TFs between geographically diverse laboratories, indicating possible differences in the human populations studied, or more likely, that laboratory practices differed in unique aspects, which introduced variability. Although the TFs significantly differed by race, it was very difficult to distinguish completely a race effect from a laboratory effect.
Nearly all studies have reported age as a strong predictor of TFs [11,12,15,23–25] but upward curvature at older ages was not consistently observed. We definitively demonstrate the presence of upward curvature at older ages in this report. The data from the Western European laboratories did not completely conform to this relationship, although an earlier report consisting of nearly all of the same laboratories noted the presence of some upward curvature [24]. An explanation for the difference was that an additional Western European laboratory was included in the present analysis and the TFs from this laboratory were lower at older ages.
The frequency of stable translocations is thought to increase with age because they are induced throughout life and undergo little negative selection during mitosis [33,39,40]. However, the reason(s) translocation frequencies increase in a curvilinear manner is unknown. Several age-related mechanisms may explain this observation. Age-related increased exposure to external clastogens may occur, e.g. diagnostic x-rays, and while this may explain part of the increase in TFs at older ages, it seems unlikely that the increase occurring between birth and age 20 is caused by medical x-ray exposure. Increases in internal clastogenic activity with age, such as reactive oxygen species, may be possible but such a relationship with the induction of chromosome exchanges has not been established. Similarly, age-related changes in double strand break repair remain largely unexplored. A decrease in the population renewal rate of T-lymphocytes with age is also possible, but again, the effect on TFs is unknown. Of relevance here is the observation that the frequency of dicentrics (having two centromeres) appears to increase with age [11,41]. Since cells with dicentrics undergo strong negative selection during mitosis [42], at steady-state their frequency will remain proportional to their rate of induction provided that the above-mentioned mechanisms are not operational. However, the fact that the frequency of dicentrics does increase with age argues in favor either for an increase in their rate of formation or a decrease in their rate of selection, or both, rendering support to one or more of the described scenarios. While at present it is not possible to distinguish between these possibilities, the dicentric data provide corroborative support for the notion that the rate of formation and/or the rate of negative selection are not constant over the human life span.
The present study is the most definitive to date to show that cigarette smoking increases TFs. The only other large pooled study on this topic of evaluated 385 individuals from Western Europe, and this study did not find a significant effect of smoking on TF [24]. Pooled studies, with very large sample sizes, may facilitate the detection of effects such as smoking on TF. In addition, we conducted multivariate modeling, i.e. we allowed for statistical adjustment of other factors, which also may have permitted us to identify a smoking effect. However, we can only speculate whether the effect of smoking was biologically meaningful. The study was not designed to evaluate specific chromosome translocations or disease outcomes, such as cancer, so a relationship between cigarette smoking and other health outcomes can only be inferred [43,44].
Gender did not affect TF, in agreement with several previous studies conducted among control populations [23,24]. We were unable to distinguish the separate effects of laboratory and race, especially with respect to differences between TFs among Asians compared to Blacks. It appeared that Blacks may have lower TFs than Whites. Future work in the same laboratory or in multiple laboratories with adherence to standardized procedures and with a robust study design will be required to determine whether the differences detected among races are real.
The age-specific background TFs provided in this report will be valuable to future studies for comparison when assessing the biological effects of environmental exposure or to provide data for sample size and power calculations. Moreover, our data could be used for comparison values in retrospective exposure studies where physical dosimetry is uncertain or unavailable.
Our pooled study from 16 international laboratories has several strengths including a sample size that was far larger than that attainable by a single institution. This allowed us to conduct statistical modeling and to carry out several sensitivity and stratified analyses to examine the consistency of the relationships we observed. Inter-laboratory differences contributed to the variability in TFs, underlining the importance of inter-laboratory comparisons, especially in samples with low translocation yields [5].
This study has several limitations. While we were able to evaluate certain specific covariates for the questionnaire data that were common among the different laboratories, these were limited to age, gender, race, and smoking status (ever/never). Future studies should consider effects of other lifestyle factors on TFs such as alcohol consumption, and should be designed to obtain more detailed smoking histories than were possible in the present, retrospective, effort. A prospective study is needed that would evaluate age, race, and other under-studied sub-groups to maximize the information gained. TFs exhibit a wide range of inter-individual variability, much of which is unexplained. Laboratory differences appear to be a significant factor but the variability could be related to underlying genotypes. A prospective study could also genotype each research subject for several haplotype-tagging variants in genes involved in DNA repair, telomere maintenance, apoptosis, cell cycle checkpoint control, and others that could help determine the source of the variability in TFs. Some efforts have already been undertaken following this line of inquiry [45–47]. The data presented here are sparse for some age-specific strata, particularly between ages 1 and 5, and 75 years or older. Another limitation concerns the possibly varying level of diagnostic radiation exposure for the populations from each of the contributing laboratories, which could affect the baseline levels of translocations since they represent cumulative exposure and may have contributed to the observation of upward curvature at older ages. However, we were unable to evaluate the effect of personal x-ray examinations in the present study.
Chromosome aberration frequencies have increasingly been used for the study of various occupational and environmental exposures [2]. The availability of a resource for comparison, such as we have provided with this pooled study of existing data, should prove beneficial. The usefulness of this resource presumes the research laboratory follows procedures that are similar to those presented here, whether the putative exposure is to radiation or to chemicals. It is clear that despite concerted attempts to reduce inter-laboratory variation, there remain unique influences of the individual laboratories that are unlikely to be resolved without addressing the reasons for the underlying variability.
In summary, we found that the relationship between age and TF was best described as linear at younger ages with upward curvature at older ages. The reason for the acceleration of TFs over age 60 is not known. The age-specific TFs were significantly modified by smoking. However, without knowing other health-related outcomes (such as cancer occurrence) it is difficult to conclude that the increased TF in ever smokers was biologically important for disease risk. We did not detect a modifying effect of gender, and the influence of race was difficult to interpret given the laboratory variation. We have provided an international resource for comparing TFs in control populations, i.e. in people who are unexposed to ionizing radiation and chemicals except for normal background levels and small medical diagnostic exposures. These data may be used in designing future studies and for evaluating instances of accidental or occupational radiation exposure.
Acknowledgments
This research was supported in part by the Intramural Research Program of the Division of Cancer Epidemiology and Genetics, the National Cancer Institute (NCI), NIH, DHHS and by an Intra-agency agreement between the National Institute of Allergy and Infectious Diseases (NIAID) and the NCI, NIAID agreement #Y2-A1-5077 and #Y3-CO-5117; performed in part under the auspices of the U.S. Department of Energy by the Lawrence Livermore National Laboratory from contract No. W-7405-ENG-48; CEC Concerted Action (FIGD-CT-200-20040; a grant from the Czech Ministry of Environment VaV/340/2/00, VaV/740/5/03 and VaV-SL/5/160/05; The Radiation Effects Research Foundation (RERF), Hiroshima and Nagasaki, Japan, is a private, non-profit foundation funded by the Japanese Ministry of Health, Labour and Welfare (MHLW) and the US Department of Energy (DOE), the latter through the National Academy of Sciences, the present publication was supported by RERF Research Protocol RP 8-93; The Spanish Nuclear Safety Council (ref 246/96); The European Union SOUL project (FIP6R-516478); a grant from the Russian Foundation for Basic Research, 01-0449118; British Nuclear Fuels plc, United Kingdom; an interagency agreement between the NCI and the National Institute for Occupational Safety and Health, Y1-CP-9012. The findings and conclusions in this report are those of the author(s) and do not necessarily represent the views of the National Institute for Occupational Safety and Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Boffetta P, van der Hel O, Norppa H, Fabianova E, Fucic A, Gundy S, Lazutka J, Cebulska-Wasilewska A, Puskailerova D, Znaor A, Kelecsenyi Z, Kurtinaitis J, Rachtan J, Forni A, Vermeulen R, Bonassi S. Chromosomal aberrations and cancer risk: results of a cohort study from Central Europe. Am J Epidemiol. 2007;165:36–43. doi: 10.1093/aje/kwj367. [DOI] [PubMed] [Google Scholar]
- 2.Bonassi S, Ugolini D, Kirsch-Volders M, Stromberg U, Vermeulen R, Tucker JD. Human population studies with cytogenetic biomarkers: review of the literature and future prospectives. Environ Mol Mutagen. 2005;45:258–270. doi: 10.1002/em.20115. [DOI] [PubMed] [Google Scholar]
- 3.Hagmar L, Bonassi S, Strömberg U, BrØgger A, Knudsen LE, Norppa H, Reuterwall C European_Study_Group_on_Cytogenetic_Biomarkers_and_Health. Chromosomal aberrations in lymphocytes predict human cancer: A report from the European study group on cytogenetic biomarkers and health (ESCH) Cancer Research. 1998;58:4117–4121. [PubMed] [Google Scholar]
- 4.Rossner P, Boffetta P, Ceppi M, Bonassi S, Smerhovsky Z, Landa K, Juzova D, Sram RJ. Chromosomal aberrations in lymphocytes of healthy subjects and risk of cancer. Environ Health Perspect. 2005;113:517–520. doi: 10.1289/ehp.6925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Edwards AA, Lindholm C, Darroudi F, Stephan G, Romm H, Barquinero J, Barrios L, Caballin MR, Roy L, Whitehouse CA, Tawn EJ, Moquet J, Lloyd DC, Voisin P. Review of translocations detected by FISH for retrospective biological dosimetry applications. Radiat Prot Dosimetry. 2005;113:396–402. doi: 10.1093/rpd/nch452. [DOI] [PubMed] [Google Scholar]
- 6.Jones IM, Galick H, Kato P, Langlois RG, Mendelsohn ML, Murphy GA, Pleshanov P, Ramsey MJ, Thomas CB, Tucker JD, Tureva L, Vorobtsova I, Nelson DO. Three somatic genetic biomarkers and covariates in radiation exposed Russian clean-up workers of the Chernobyl nuclear reactor, 6 – 13 years after exposure. Radiation Research. 2002;158:424–442. doi: 10.1667/0033-7587(2002)158[0424:tsgbac]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 7.Kleinerman RA, Littlefield LG, Tarone RE, Sayer AM, Hildreth NG, Pottern LM, Machado SG, Boice JJD. Chromosome aberrations in relation to radiation dose following partial-body exposures in three populations. Radiation Research. 1990;123:93–101. [PubMed] [Google Scholar]
- 8.Tawn EJ, Whitehouse CA. Persistence of translocation frequencies in blood lymphocytes following radiotherapy: implications for retrospective radiation biodosimetry. J Radiol Prot. 2003;23:423–430. doi: 10.1088/0952-4746/23/4/005. [DOI] [PubMed] [Google Scholar]
- 9.Tucker JD. FISH cytogenetics and the future of radiation biodosimetry. Radiation Protection Dosimetry. 2001;97:55–60. doi: 10.1093/oxfordjournals.rpd.a006638. [DOI] [PubMed] [Google Scholar]
- 10.Pressl S, Edwards A, Stephan G. The influence of age, sex and smoking habits on the background level of FISH-detected translocations. Mutation Research. 1999;442:89–95. doi: 10.1016/s1383-5718(99)00067-4. [DOI] [PubMed] [Google Scholar]
- 11.Ramsey MJ, Moore DH, II, Briner JF, Lee DA, Olsen LA, Senft JR, Tucker JD. The effects of age and lifestyle factors on the accumulation of cytogenetic damage as measured by chromosome painting. Mutation Res. 1995;338:95–106. doi: 10.1016/0921-8734(95)00015-x. [DOI] [PubMed] [Google Scholar]
- 12.Tucker JD, Lee DA, Ramsey MJ, Briner J, Olsen L, Moore DH., II On the frequency of chromosome exchanges in a control population measured by chromosome painting. Mutation Research. 1994;313:193–202. doi: 10.1016/0165-1161(94)90049-3. [DOI] [PubMed] [Google Scholar]
- 13.van Diemen PC, Maasdam D, Vermeulen S, Darroudi F, Natarajan AT. Influence of smoking habits on the frequencies of structural and numerical chromosomal aberrations in human peripheral blood lymphocytes using the fluorescence in situ hybridization (FISH) technique. Mutagenesis. 1995;10:487–495. doi: 10.1093/mutage/10.6.487. [DOI] [PubMed] [Google Scholar]
- 14.Vorobtsova I, Semenov A, Timofeyeva N, Kanayeva A, Zvereva I. An investigation of the age-dependency of chromosome abnormalities in human populations exposed to low-dose ionising radiation. Mech Ageing Dev. 2001;122:1373–1382. doi: 10.1016/s0047-6374(01)00275-5. [DOI] [PubMed] [Google Scholar]
- 15.Vorobtsova IE, Tucker JD, Timofeeva NM, Bogomazova AN, Semenov AV, Pleshanov PG. Effect of age and radiation exposure on the frequency of translocations and dicentrics detected by FISH method in human lymphocytes [Article in Russian] Radiat Biol Radioecol. 2000;40:142–148. [PubMed] [Google Scholar]
- 16.Bender M, Preston R, Leonard R, Pyatt B, Gooch P. Chromosomal aberration and sister-chromatid exchange frequencies in peripheral blood lymphocytes of a large human population sample. II. Extension of age range. Mutat Res. 1989;212:149–154. doi: 10.1016/0027-5107(89)90065-1. [DOI] [PubMed] [Google Scholar]
- 17.Galloway SM, Berry PK, Nichols WW, Wolman SR, Soper KA, Stolley PD, Archer P. Chromosome aberrations in individuals occupationally exposed to ethylene oxide, and in a large control population. Mutat Res. 1986;170:55–74. doi: 10.1016/0165-1218(86)90082-0. [DOI] [PubMed] [Google Scholar]
- 18.Prieur M, Al Achkar W, Aurias A, Couturier J, Dutrillaux AM, Dutrillaux B, Flury-Herard A, Gerbault-Seureau M, Hoffschir F, Lamoliatte E, Lefrancois D, Lombard M, Muleris M, Ricoul M, Sabatier L, Viegas-Pequignot E. Acquired chromosome rearrangements in human lymphocytes: effect of aging. Hum Genet. 1988;79:147–150. doi: 10.1007/BF00280554. [DOI] [PubMed] [Google Scholar]
- 19.Tawn EJ. The frequency of chromosome aberrations in a control population. Mutat Res. 1987;182:303–308. doi: 10.1016/0165-1161(87)90072-0. [DOI] [PubMed] [Google Scholar]
- 20.Tawn EJ, Whitehouse CA. Frequencies of chromosome aberrations in a control population determined by G banding. Mutation Research. 2001;490:171–177. doi: 10.1016/s1383-5718(00)00164-9. [DOI] [PubMed] [Google Scholar]
- 21.ICRU. Retrospective assessment of exposures to ionizing radiation. Nuclear Technology Publishing; 2002. International Commission on Radiation Units and Measurements (ICRU) report 68; p. 48.p. 50. [Google Scholar]
- 22.Sorokine-Durm I, Durand V, Delbos M, Baron L, Roy L, Voisin P. A French view on FISH painting as a biodosemeter. Radiation Protection Dosimetry. 2000;88:35–44. [Google Scholar]
- 23.Sorokine-Durm I, Whitehouse C, Edwards A. The variability of translocation yields amongst control populations. Radiation Protection Dosimetry. 2000;88:93–99. [Google Scholar]
- 24.Whitehouse CA, Edwards AA, Tawn EJ, Stephan G, Oestreicher U, Moquet JE, Lloyd DC, Roy L, Voisin P, Lindholm C, Barquinero J, Barrios L, Caballin MR, Darroudi F, Fomina J. Translocation yields in peripheral blood lymphocytes from control populations. Int J Radiat Biol. 2005;81:139–145. doi: 10.1080/09553000500103082. [DOI] [PubMed] [Google Scholar]
- 25.Lucas JN, Deng W, Moore D, Hill F, Wade M, Lewis A, Sailes F, Kramer C, Hsieh A, Galvan N. Background ionizing radiation plays a minor role in the production of chromosome translocations in a control population. Int J Radiat Biol. 1999;75:819–827. doi: 10.1080/095530099139872. [DOI] [PubMed] [Google Scholar]
- 26.Fomina J, Darroudi F, Boei J, Natarajan A. Discrimination between complete and incomplete chromosome exchanges in X-irradiated human lymphocytes using FISH with pan-centromeric and chromosome specific DNA probes in combination with telomeric PNA probe. Int J Radiat Biol. 2000;76:807–813. doi: 10.1080/09553000050028968. [DOI] [PubMed] [Google Scholar]
- 27.Cigarran S, Barquinero JF, Barrios L, Ribas M, Egozcue J, Caballin MR. Cytogenetic analyses by fluorescence in situ hybridization (FISH) in hospital workers occupationally exposed to low levels of ionizing radiation. Radiat Res. 2001;155:417– 423. doi: 10.1667/0033-7587(2001)155[0417:cabfis]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 28.Livingston GK, Falk RB, Schmid E. Effect of occupational radiation exposures on chromosome aberration rates in former plutonium workers. Radiat Res. 2006;166:89–97. doi: 10.1667/RR3586.1. [DOI] [PubMed] [Google Scholar]
- 29.Nakano M, Kodama Y, Ohtaki K, Itoh M, Awa AA, Cologne J, Kusunoki Y, Nakamura N. Estimating the number of hematopoietic or lymphoid stem cells giving rise to clonal chromosome aberrations in blood T lymphocytes. Radiat Res. 2004;161:273–281. doi: 10.1667/rr3133. [DOI] [PubMed] [Google Scholar]
- 30.Oestreicher U, Braselmann H, Stephan G. Cytogenetic analyses in peripheral lymphocytes of persons living in houses with increased levels of indoor radon concentrations. Cytogenet Genome Res. 2004;104:232–236. doi: 10.1159/000077495. [DOI] [PubMed] [Google Scholar]
- 31.Roy L, Gregoire E, Durand V, Buard V, Delbos M, Paillole N, Sorokine-Durm I, Gourmelon P, Voisin P. Study of the tools available in biological dosimetry to estimate the dose in cases of accidental complex overexposure to ionizing radiation: the Lilo accident. Int J Radiat Biol. 2006;82:39–48. doi: 10.1080/09553000600579207. [DOI] [PubMed] [Google Scholar]
- 32.Sram RJ, Rossner P, Rubes J, Beskid O, Dusek Z, Chvatalova I, Schmuczerova J, Milcova A, Solansky I, Bavorova H, Ocadlikova D, Kopecna O, Musilova P. Possible genetic damage in the Czech nuclear power plant workers. Mutat Res. 2006;593:50–63. doi: 10.1016/j.mrfmmm.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 33.Tucker JD, Cofield J, Matsumoto K, Ramsey MJ, Freeman DC. Persistence of chromosome aberrations following acute radiation: I, PAINT translocations, dicentrics, rings, fragments, and insertions. Environ Mol Mutagen. 2005;45:229–248. doi: 10.1002/em.20090. [DOI] [PubMed] [Google Scholar]
- 34.Zhang W, Wang C, Chen D, Minamihisamatsu M, Morishima H, Yuan Y, Wei L, Sugahara T, Hayata I. Imperceptible effect of radiation based on stable type chromosome aberrations accumulated in the lymphocytes of residents in the high background radiation area in China. J Radiat Res (Tokyo) 2003;44:69–74. doi: 10.1269/jrr.44.69. [DOI] [PubMed] [Google Scholar]
- 35.Zhang W, Wang C, Chen D, Minamihisamatsu M, Morishima H, Yuan Y, Wei L, Sugahara T, Hayata I. Effect of smoking on chromosomes compared with that of radiation in the residents of a high-background radiation area in China. J Radiat Res (Tokyo) 2004;45:441–446. doi: 10.1269/jrr.45.441. [DOI] [PubMed] [Google Scholar]
- 36.Tucker JD, Breneman JW, Briner JF, Eveleth GG, Langlois RG, Moore DH., 2nd Persistence of radiation-induced translocations in rat peripheral blood determined by chromosome painting. Environ Mol Mutagen. 1997;30:264–272. doi: 10.1002/(sici)1098-2280(1997)30:3<264::aid-em4>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 37.Morton N. Parameters of the human genome. Proc Natl Acad Sci. 1991;88:7474–7476. doi: 10.1073/pnas.88.17.7474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Preston D, Lubin J, Pierce D, McConney M. Epicure Release 2.0. HiroSoft International Corporation; Seattle: 1996. [Google Scholar]
- 39.Gardner SN, Tucker JD. The cellular lethality of radiation-induced chromosome translocations in human lymphocytes. Radiation Research. 2002;157:539–552. doi: 10.1667/0033-7587(2002)157[0539:tclori]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 40.Matsumoto K, Ramsey MJ, Nelson DO, Tucker JD. Persistence of radiation-induced translocations in human peripheral blood determined by chromosome painting. Radiation Research. 1998;149:602–613. [PubMed] [Google Scholar]
- 41.Tonomura A, Kishi K, Saito F. Types and frequencies of chromosome aberrations in peripheral lymphocytes of general populations. In: Ishihara T, Sasaki M, editors. Radiation-induced chromosome damage in man. New York: 1983. pp. 605–616. [Google Scholar]
- 42.Carrano A. Chromosome aberrations and radiation induced cell death II. Predicted and observed cell survival. Mutat Res. 1973;17:355–366. doi: 10.1016/0027-5107(73)90007-9. [DOI] [PubMed] [Google Scholar]
- 43.Crane MM, Strom SS, Halabi S, Berman EL, Fueger JJ, Spitz MR, Keating MJ. Correlation between selected environmental exposures and karyotype in acute myelocytic leukemia. Cancer Epidemiol Biomarkers Prev. 1996;5:639–644. [PubMed] [Google Scholar]
- 44.Sandler DP, Shore DL, Anderson JR, Davey FR, Arthur D, Mayer RJ, Silver RT, Weiss RB, Moore JO, Schiffer CA, et al. Cigarette smoking and risk of acute leukemia: associations with morphology and cytogenetic abnormalities in bone marrow. J Natl Cancer Inst. 1993;85:1994–2003. doi: 10.1093/jnci/85.24.1994. [DOI] [PubMed] [Google Scholar]
- 45.Kiuru A, Lindholm C, Heilimo I, Ceppi M, Koivistoinen A, Ilus T, Hirvonen A, Norppa H, Salomaa S. Influence of DNA repair gene polymorphisms on the yield of chromosomal aberrations. Environ Mol Mutagen. 2005;46:198–205. doi: 10.1002/em.20155. [DOI] [PubMed] [Google Scholar]
- 46.Pluth JM, Nelson DO, Ramsey MJ, Tucker JD. The relationship between genotype and chromosome aberration frequencies in a normal adult population. Pharmacogenetics. 2000;10:311–319. doi: 10.1097/00008571-200006000-00005. [DOI] [PubMed] [Google Scholar]
- 47.Wilding CS, Relton CL, Rees GS, Tarone RE, Whitehouse CA, Tawn EJ. DNA repair gene polymorphisms in relation to chromosome aberration frequencies in retired radiation workers. Mutat Res. 2005;570:137–145. doi: 10.1016/j.mrfmmm.2004.11.002. [DOI] [PubMed] [Google Scholar]