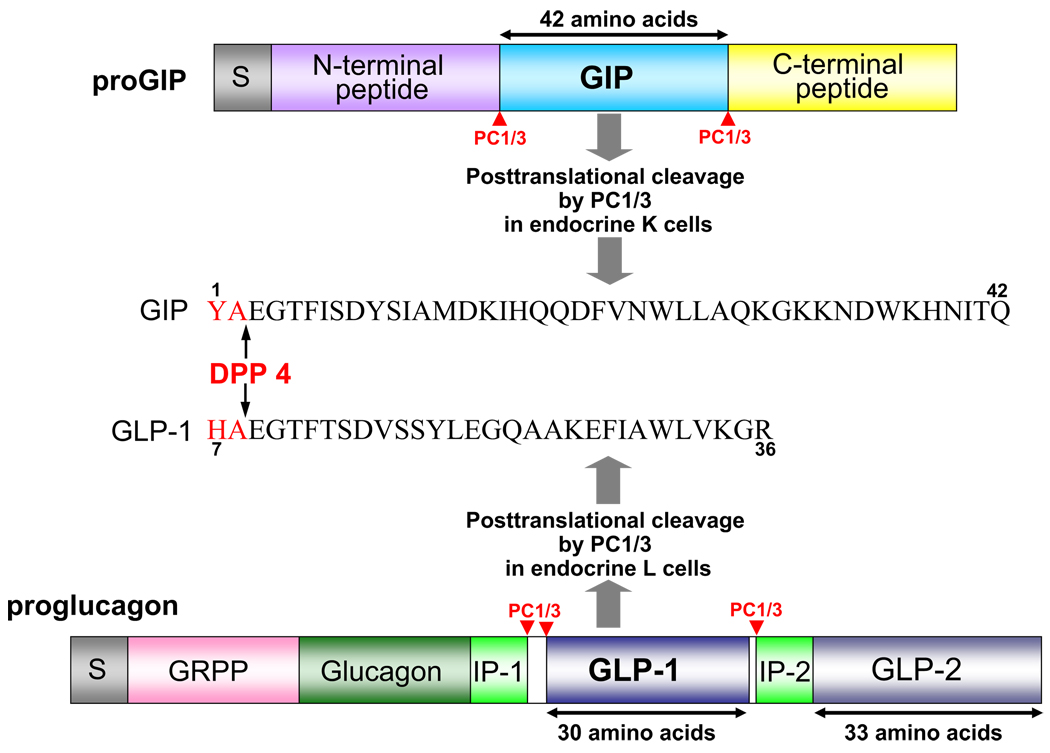
FIG. 2.
Schematic representation of proGIP and proglucagon. GIP is a single 42-amino acid peptide derived from the post-translational processing of proGIP by PC1/3 in enteroendocrine K cells. It is the only functional proGIP product in all species examined to date, and there is a greater than 90% amino acid identity between human, rat, murine, porcine, and bovine sequences. GLP-1 is a post-translational cleavage product of the proglucagon gene by PC1/3 in enteroendocrine L cells and GLP-1 (7–36) amide is a major form of circulating biologically active GLP-1 in humans. In mammals, proglucagon is expressed in pancreas, enteroendocrine L cells, brain, and taste cells with an identical mRNA transcript in each tissue. Fish and bird proglucagon mRNA in pancreas and liver, however, have different 3′-ends because of differential splicing upon pancreatic expression. The Xenopus laevis proglucagon gene encodes three unique GLP-1-like peptides, each with insulinotropic properties that are capable of activating the human GLP-1R, and one of which seems more potent than human GLP-1 (Irwin et al., 1997).