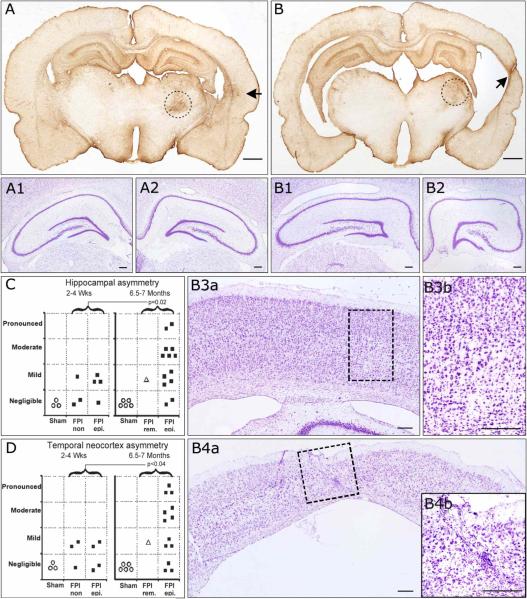
Figure 6. Progressive hippocampal and temporal cortex pathology in posttraumatic epileptic rat.
Coronal sections obtained from bregma −4 mm through bregma −5 mm and stained for cresyl violet and GFAP. A) GFAP immunoreactivity of an epileptic animal 7 months post-injury demonstrating no hippocampal and mild temporal cortex asymmetry. At higher magnification, cresyl violet staining shows symmetric contralateral (A1) and ipsilateral (A2) hippocampi and no loss of laminar features. B) GFAP immunoreactivity of another epileptic animal 7 months post-injury demonstrating pronounced hippocampal and temporal cortex asymmetry. At higher magnification, cresyl violet staining shows asymmetric contralateral (B1) and ipsilateral (B2) hippocampi, with evident atrophy of ipsilateral CA3 and CA1subregions. The contralateral temporal cortex showed no atrophy (B3a) and normal laminar structure (B3b). However, the ipsilateral temporal cortex was atrophic (B4a) and showed pronounced loss of neurons and laminar features, and increased small nuclei representing reactive glia (B4b), all typically associated with temporal cortex sclerosis. Hippocampal (C) and temporal cortex (D) asymmetry increased over time post-injury in the population of FPI animals. Statistics with one-tailed Mann-Whitney U test. FPI rem. = case of PTE remission following FPI. FPI epi. = persistently epileptic animals. FPI n.epi. = not epileptic animals. Scale bars: 1mm for A and B; 250 μm for A1−2 and B1-B4b. Black arrows in A and B indicate temporal foci of glial reactivity. Dotted circles in A and B delineate the thalamic injury present in all rpFPI animals. Dotted rectangles in B3a and B4a delineate the areas magnified in B3b and B4b, respectively.