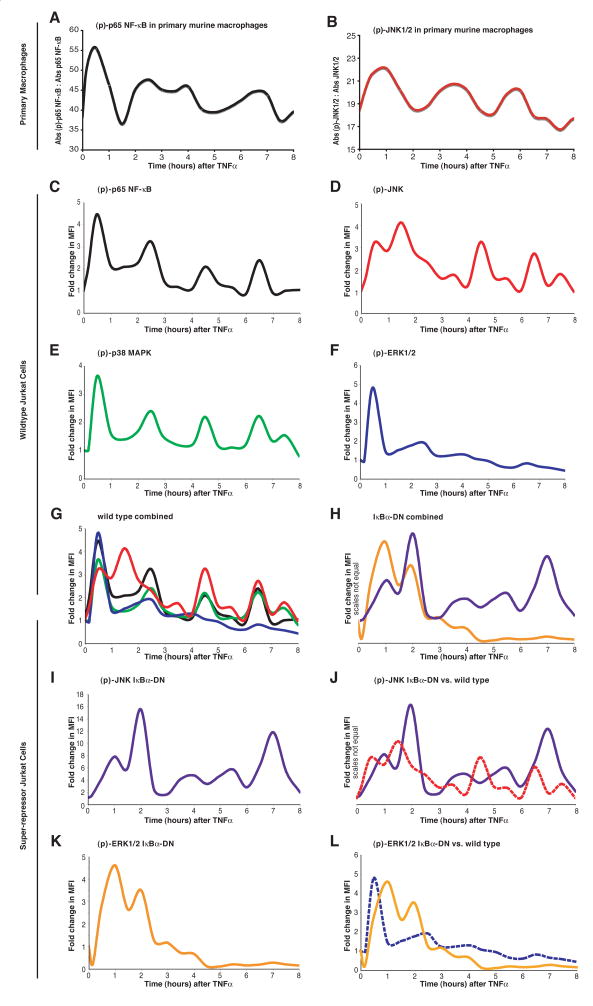
Figure 2. Phosphorylation of Signal Transduction Molecules is Oscillatory.
Temporal profiles at 30 minute intervals for 8 hours (with 10 and 20 minutes time points added) for p65 NF-κB and JNK1/2 phosphorylation in purified murine macrophages (A and B) using phospho-ELISAs following TNF addition (40 ng/mL). Data is plotted as absorbance (Abs) of the phosphorylated to non-phosphorylated protein (three replicate estimations per time point). In separate experiments, Jurkat cells were stimulated with TNF and fixed with formaldehyde at 10 minutes and at 30-minute intervals thereafter for 8 hours. After permeablization in methanol, the cells were stained for (p)-JNK1/2-Ax647 and (pS536)-p65-Ax488 or (p)-ERK1/2-Ax647 and (p)-p38 MAPK-Ax488 and analyzed by flow-cytometry to calculate the fold change in median fluorescence intensity (MFI). Increases in (pS536)-p65 (C) mirrored that of (p)-p38 MAPK (E), except that the trough for (pS536)-p65 remained significantly elevated in between the first two peaks. JNK1/2 phosphorylation (D) displayed a unique pattern for the first 3 hours, but thereafter became in unison with the phosphorylation pattern of p38 MAPK and p65. The phosphorylation of ERK1/2 (F) did not display oscillations, but rather underwent one wave of activation. The only time where all pathways were significantly activated was the initial signaling wave (G). Panel H shows similar data for JNK and ERK1/2 phosphorylation in Jurkat cells stably overexpressing the IκB super-repressor; these individual data are plotted together with that from wild type vector-transfected cells (I and J). Please refer to Supplementary Figure 2 for a representative plot, including a raw data set, from the flow cytometry experiments showing the calculation of a change in MFI at each time point.