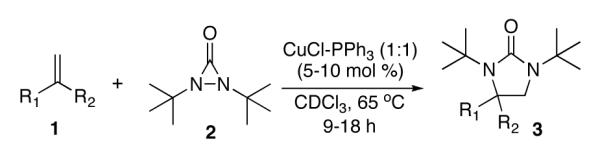
Table 1.
Catalytic Diamination of Disubstituted Terminal Olefinsa
 | |||
|---|---|---|---|
| entry | substrate 1 | product 3 | yield (%)d |
 |
 |
||
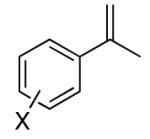
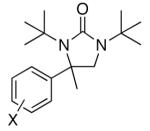
| 1 | 1a, X = H | 91 | |
| 2 | 1b, X = o-F | 47 | |
| 3b | 1c, X = o-OMe | 50 | |
| 4 | 1d, X = m-Br | 86 | |
| 5 | 1e, X = m-OMe | 74 | |
| 6 | 1f, X = m-Me | 83 | |
| 7 | 1g, X = m-CN | 80 | |
| 8 | 1h, X = p-Me | 64 | |
| 9 | 1i, X = p- Cl | 90 | |
| 10c |
 lj |
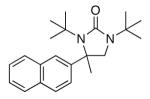 |
86 |
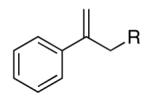 |
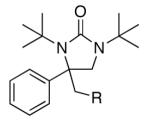 |
||
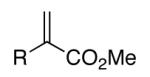
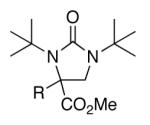
| 11 | 1k, R = Me | 71 | |
| 12 | 1l, R = Ph | 66 | |
| 13b | 1m, R = OMe | 63 | |
| 14 |
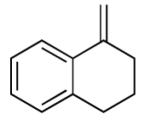 ln |
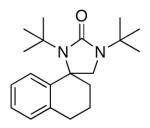 |
55 |
 |
 |
||
| 15 | 1o, R = Ph | 65 | |
| 16 | 1p, R = Me | 54 | |
All reactions were carried out with olefin (1) (0.4 mmol), di-tert-butyldiaziridinone (2) (0.8 mmol) (added by syringe pump over 8 h), CuCl-PPh3 (1:1) (0.02 mmol) in CDCl3 (0.3 mL) at 65 °C unless otherwise stated. Upon complete addition of 2, the reaction mixture was stirred at 65 °C for an additional time period (1 h for entries 1, 4, 5, 6, 8, and 9; 2 h for entries 7, 10, and 15; 4 h for entry 11; 7 h for entries 3, 12, 14, and 16; 10 h for entries 2 and 13).
CuCl-PPh3 (1:1) (0.04 mmol) was used.
The reaction was carried out with olefin (0.2 mmol), di-tert-butyldiaziridinone (2) (0.4 mmol), and CuCl-PPh3 (1:1) (0.02 mmol) in CDCl3 (0.3 mL).
Isolated yield based on olefin.
