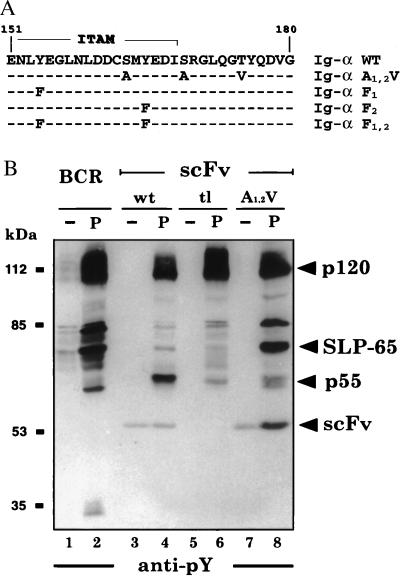
Figure 1.
Mutation of the serine (S) and threonine (T) residues in the Ig-α tail increases the signal transduction from chimeric scFv/Ig-α molecules. (A) Position of the two serines and the threonine in the cytoplasmic sequence of Ig-α. The ITAM sequence is overlined. The mutations are shown in bold, and dashes indicate the identity to the wild-type Ig-α sequence. (B) PTK substrate phosphorylation in activated J558L B cells expressing the complete BCR (lanes 1 and 2) or chimeric scFv/Ig-α molecules with the wild-type Ig-α tail sequence (wt, lanes 3 and 4), a cytoplasmic sequence of only five amino acids, RRIDP (tl, lanes 5 and 6), or the A1,2V-mutated Ig-α tail (A1,2V, lanes 7 and 8). The cells were either left untreated (lanes 1, 3, 5, and 7) or exposed for 2 min to 20 μM pervanadate (lanes 2, 4, 6, and 8). Proteins of total cellular lysates were size-separated by SDS/10% PAGE and analyzed by Western blotting with the anti-pY antibody 4G10.