Abstract
An adult, male, rhesus macaque presented with pruritus and a focal, exudative, inflamed, erythematous skin lesion of approximately 2 cm in diameter on the ventral aspect of the mandible. The lesion resolved after 10 d of treatment with 1% chlorhexidine solution and triple-antibiotic ointment. However, the skin lesion subsequently recurred several times over a 2-mo period. A punch biopsy was performed, and histological changes were most consistent with a diagnosis of atopic dermatitis. Treatment with topical tacrolimus ointment, an immunosuppressive drug, proved successful in the resolution of all clinical signs after 4 mo. According to a literature review, this article is the first report of the use of tacrolimus ointment as a topical treatment of atopic dermatitis in a rhesus macaque.
Abbreviation: AD, atopic dermatitis
Atopic dermatitis (AD) is a chronic inflammatory skin disease characterized by pruritus and a distinctive age-related distribution of skin lesions.22 This disease typically is thought to develop subsequent to exposure to irritant or allergen, resulting in maculopapular dermatitis. The areas usually involved are sparsely haired. Diagnosis is based on history, clinical signs, histopathology when possible, and patch testing in humans.16 The lesion in the rhesus macaque we present was localized to a single area, similar to the onset of disease seen in adult humans,11,12 but different from previously reported cases in juvenile nonhuman primates16,18 representative of pediatric cases.27 Atopic dermatitis affects 1 in 5 Americans and is the leading cause of chronic skin disease in children. Symptoms in humans usually begin at approximately 5 y of age but may arise in younger children, and clinical signs are seen into adulthood.23 Topical corticosteroids traditionally have been used to treat AD, but the utility of these drugs as a long-term treatment option is mitigated by their known side-effects.22 Recent use of antihistamines, emollients, and immunosuppressants as treatment options for AD in humans has reduced the use of corticosteroids.22,23
Tacrolimus, a macrolide lactone of fungal origin (Streptomyces tsukubaensis), is an immunosuppressive drug commonly used in humans.2,23,33 This drug initially was used intravenously in organ transplant patients. Later, tacrolimus was developed as a topical ointment, and in 2000, the US Food and Drug Administration approved the use of topical tacrolimus as a treatment for AD.22
AD has only rarely been documented to occur in nonhuman primates, with rhesus macaques (Macaca mulatta) and cynomolgus macaques (Macaca fascicularis) implicated in the few published cases.15,16,18 In 1 recent case report, the immunosuppressive drug cyclosporine was used systemically to treat a case of AD in a rhesus macaque.18 Using this rationale, we successfully treated a case of AD in an adult rhesus macaque with topical tacrolimus ointment. We felt that systemic treatment with other conventional drugs used for AD treatment was unnecessary and inappropriate in this case, because the lesion was localized and because systemic drugs were incompatible with the ongoing research project. To our knowledge, this article is the first report of the successful long-term treatment of atopic dermatitis with topical tacrolimus in a rhesus macaque.
Case report
The patient was an adult male rhesus macaque (Macaca mulatta) estimated to be older than 10 y that was in good body condition and weighed 11.8 kg. He was singly housed at a facility fully accredited by AAALAC. The macaque had visual access to its conspecifics and was provided with various manipulanda and treats according to institutional guidelines on environmental enrichment for nonhuman primates. The animal was enrolled in a protocol that was approved by the Institutional Animal Care and Use Committee and had been in the same laboratory and facility for 8 y without any reports of skin disease. This macaque was used for simultaneous behavioral and electrophysiologic experiments. The team gained insight into the relationship between neuronal activity and behavioral capacities by means of a head implant with microarray electrodes by which neuronal activity during the performance of various tasks was recorded. Routine husbandry parameters were 12:12-h light:dark cycles; controlled temperature, humidity, and ventilation (18 to 29 °C, 30% to 70%, and 10 to 15 air exchanges per hour, respectively); commercial primate chow (Monkey Diet 5038 Lab Diet, PMI Nutrition International, St Louis, MO); and daily supplementation with a variety of fresh fruits and vegetables. No changes in the diet or environment had occurred during the last 12 mo. Sanitation consisted of complete cage changes every 2 wk and replacement of litter (Soft-Texture Paper Chip, Shepherd Specialty Papers Chicago, IL) 3 times per week. The macaque did not have any known direct contact with chemicals or detergents. The animal was seronegative for Cercopithecine herpesvirus 1, SIV, simian T-lymphotropic virus, and simian retrovirus type D and tested negative for Mycobacterium tuberculosis (TB) by both an intradermal mammalian tuberculin test (Synbiotics Corporation, San Diego, CA) and by a cell-mediated immunity assay (Primagam, Prionics AG, Switzerland). These tests were performed during quarantine, and in addition, serologic testing for Cercopithecine herpesvirus 1 was performed annually and tuberculosis testing was quarterly. The animal was otherwise healthy without any known medical problems.
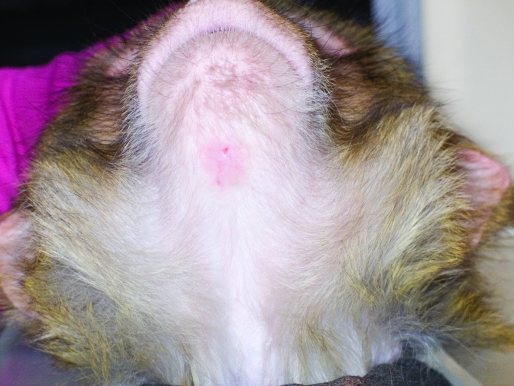
The rhesus macaque presented with a focal, exudative, erythematous, raised lesion (diameter, 2 cm) of the sparsely haired skin near the ventral mandibular symphysis. Alopecia, scaling, lichenification, and exfoliation were present within and around the skin lesion site (Figure 1). The site was mildly pruritic.
Figure 1.
Adult male rhesus macaque with focal, exudative, erythematous, raised skin lesion of approximately 2 cm in diameter in the sparsely haired skin near the ventral mandibular symphysis under the chin. Alopecia, scaling, lichenification, and exfoliation are present at the papule site and its immediate surroundings.
Initial differential diagnosis included: behavioral causes (self-trauma), environmental causes (humidity level, diet, or environmental changes), bacterial infection (primary or secondary), fungal infection, parasitic infection, allergy, and atopic dermatitis. Less likely causes that were also considered were nutritional deficiencies, endocrinopathies, and neoplasia. Local treatment with 1% chlorhexidine solution (Nolvasan, Fort Dodge Animal Health, Fort Dodge, IA) was initiated immediately, and an ophthalmic ointment containing bacitracin, neomycin, and polymyxin B (BNP ophthalmic ointment, Pharmaderm, Animal Health, Melville, NY) applied twice daily for 10 d. No chemical restraint was necessary—once chaired, the macaque readily allowed local treatment. In addition, after a few days, the macaque was trained to present his chin for treatment, making chairing unnecessary. The condition resolved, only to relapse 3 d later after cessation of treatment.
An ointment combining neomycin sulfate, thiabendazole, and dexamethasone (Tresaderm, Merial Limited, Duluth, GA) was used twice daily for 2 d but was discontinued due to worsening of the symptoms. Treatment was effective with the application of a cream containing nystatin, neomycin sulfate, thiostrepton, and triamcinolone acetonide (Panolog, Novartis Animal Health Canada, Mississauga, Ontario) twice daily for 14 d. However, the lesion recurred 3 d later. Treatment was repeated for 5 d, leading to resolution and cessation of treatment. Five weeks later, the lesion returned at the same location. The next day, the macaque was fasted and sedated with ketamine (10 mg/kg IM; Vetaket, Lloyd, Shenandoah, IA) for physical examination and multiple diagnostic tests, including a complete blood count, a serum chemistry panel, skin scrapings for ectoparasites detection, hair plucks for fungal culture, and histopathologic examination of full-thickness skin punch biopsy (diameter, 4 mm) from affected and nonaffected skin areas.
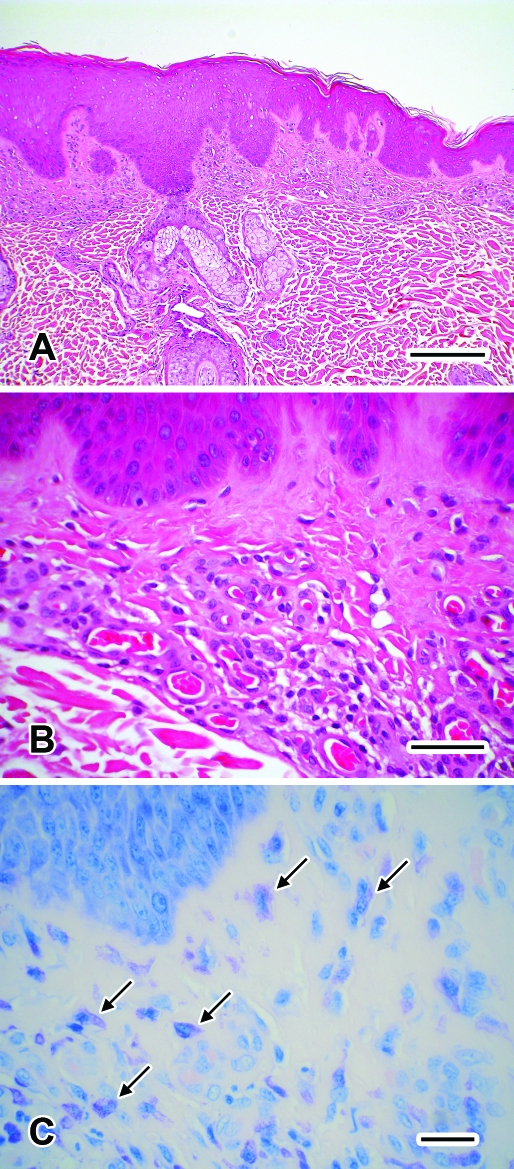
All tests were performed inhouse and all, except for the biopsy, were within normal limits or negative. Histologic examination of tissue sections stained with hematoxylin and eosin or toluidine blue (Figure 2) revealed the presence of moderate numbers of mast cells distributed diffusely within the superficial dermis, moderate superficial dermal fibrosis, and moderate thickening of the walls of superficial dermal vessels. Overlying epidermis was moderately thickened with acanthosis. Acid-fast and tissue Gram stains failed to reveal mycobacteria and bacterial agents, respectively, within the sections. There was no evidence for other pathology, such as neoplasia, fungal infection, and atrophic and nutritional dermatoses. The pathologist concluded that the clinical signs and microscopic changes were most consistent with a diagnosis of atopic dermatitis.
Figure 2.
Biopsy of the skin lesion from the sparsely haired-skin of the ventral mandible from the rhesus macaque from Figure 1. (A) Diffuse acanthosis of the epidermis. Hematoxylin and eosin stain; bar, 200 μm. (B) Moderate superficial dermal fibrosis and moderate thickening of the walls of superficial dermal vessels. Hematoxylin and eosin stain; bar, 50 μm. (C) Moderate numbers of mast cells (arrows) are noted with a diffuse superficial dermal distribution. Toluidine blue stain; bar, 25 μm.
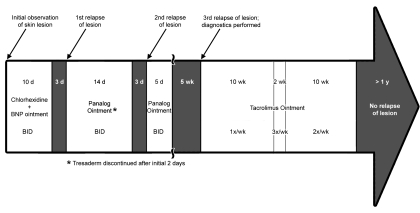
In light of the mixed success and long-term side effects of topical corticosteroids and restrictions placed by the research protocol, we searched for a topical immunosuppressant and consulted with a human dermatologist. Accordingly tacrolimus ointment at 0.1% (Protopic, Astellas Pharma US, Deerfield, IL) was applied to the lesion daily for 10 wk. Subsequently, the treatment frequency was decreased to 3 times a week for 2 wk, followed by 2 times a week for 2 wk. When the frequency of treatment was decreased to once weekly, the lesion began to recur. Therefore, treatment was maintained at 2 applications a week for 8 wk until complete regression of the lesion, at which point the treatment was discontinued. The goal was to reduce the exposure to tacrolimus to a minimum while appropriately treating the lesion. At the time, this schedule was not based on any published recommended guidelines for the use of tacrolimus in humans, but rather was a logical consequence of the possible risk inherent to the use of calcineurin inhibitors. However, this method proved to be similar to that used in the treatment of adult human patients with AD.33 No clinically observable side-effects were noted during the period of tacrolimus administration in this macaque. The skin lesion had not recurred at the time of submission of this case report, more than 1 y after cessation of treatment (Figure 3).
Figure 3.
Clinical course of the atopic dermatitis case.
Discussion
The use of rhesus macaques as a model of AD was first proposed in 1986, when application of 5% nicotinate ointment to the sexual skin (glabrous skin) and shaved haired skin led to an atopic reaction of the sexual skin in 5 to 10 min after application, as compared with the haired skin, which remained normal and reaction-free.32 Several other studies show that the sparsely haired skin in humans and animals often is involved in AD.8-11,16,18,23 In dogs and cats, the haircoat is thought to be protective against potential allergens.8 The same explanation could be inferred for humans and nonhuman primates. Glabrous skin usually is thinner than haired skin, and the long-term treatment of glabrous skin must take this difference into account. Therefore, long-term topical use of corticosteroids, known to induce skin atrophy and reduce the innate barrier effect of the skin (as well as other systemic side effects), is discouraged in the head, neck, and sensitive skin regions in humans.14 In our experience, initial topical corticosteroids and antibiotics proved helpful at controlling the AD lesion of the macaque we describe. The location (ventral aspect of the mandible), distribution and extent (focal), and research constraints limited the treatment options to conservative topical therapy. After consultation with a human dermatologist, treatment with tacrolimus was proposed. In addition, tacrolimus is known to be effective as a topical treatment of AD in humans, reinforcing our decision to evaluate this novel compound.1,6,17,27,33 Minimal side effects, availability in topical form, little if any systemic absorption, and potential use on mucous membranes and sensitive skin areas are advantages in using tacrolimus as a treatment for AD.23
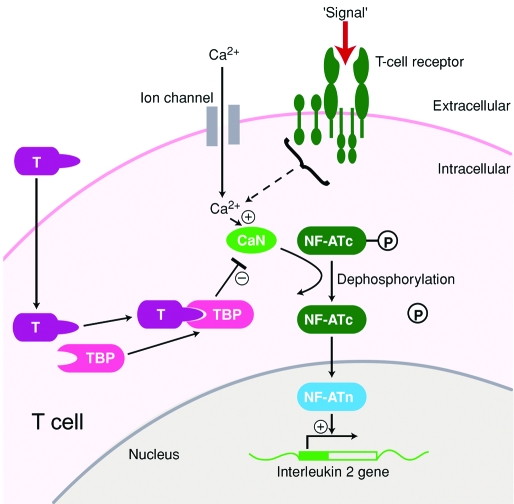
The detailed mechanism of action of tacrolimus in AD is unknown. However, its general mechanism of action is similar to that of cyclosporine, a calcineurin inhibitor (Figure 4). In addition, tacrolimus has been shown to inhibit the granules release of preformed mediators from skin mast cells and basophils and to downregulate the expression of FcϵRI (a high-affinity receptor for IgE expressed on mast cells, basophils, and Langerhan cells).2
Figure 4.
Mechanism of action of tacrolimus. In the cytoplasm, tacrolimus (T) binds to tacrolimus-binding protein (TBP), forming a tacrolimus–TBP complex, which binds to and blocks calcineurin (CaN). The T–TBP–CaN complex inhibits the activation of the nuclear factor of activated T cells (NF-ATc), thus preventing the entry of NF-ATc into the nucleus. Unable to bind to its nuclear component (NF-ATn), NF-ATc cannot bind to the promoter of the IL2 gene and initiate IL-2 production. T cells therefore will not produce IL2, which is necessary for full T-cell activation. (Modified from reference 28).
The main limitations to tacrolimus administration include: the ability to provide topical treatment, possible secondary infection due to its immunosuppressive effect (refuted27), and the warning from the US Food and Drug Administration regarding the possible risk of skin cancer.22 Although a causal relationship has not been established, the Food and Drug Administration warned in 2006 that rare cases of malignancies (for example, skin and lymphoma) developed in human patients treated with topical calcineurin inhibitors.2,22 The warning was mainly due to the lack of long-term studies. Sun exposure was 1 of the risk factors for the possible malignant effect of tacrolimus, leading the Food and Drug Administration to recommend the use of broad-spectrum sun block to all sunlight-exposed skin.22 At this point, several studies have refuted the risk of skin cancer after local treatment with tacrolimus.13,19,21,22,24,25 Regardless, our macaque was housed indoors.
Apart from being a primary treatment for AD, tacrolimus might play a role in diminishing the risk factors of asthma. Although, the link between AD and asthma is not commonly made in medicine, several reports demonstrate a likely association between asthma and AD in humans,7,29 rhesus macaques20 and dogs,4 and AD might be a precursor sign of asthma.7,29 Both conditions are the result of chronic inflammation related to disturbances of the immune system, and similar treatments are often used.7 The pathogenesis of AD is not well understood, and several predisposing factors—genetic background, environment, infectious agents, epidermal barrier dysfunction, and immunologic responses—might interact to lead to AD.19 Moreover, topical tacrolimus used to treat onset of AD seems able to ameliorate the respiratory symptoms in bronchial hyperresponsiveness, a pathophysiologic feature of asthma.29 One hypothesis is that Th2 cells may migrate from the skin to the airways.29 Therefore, tacrolimus could potentially prevent or limit the clinical signs of asthma. In addition, tacrolimus indirectly may improve the barrier function of the skin.29 By reducing inflammation, tacrolimus might decrease the absorption of antigens and prevent their systemic effects on the airways.29
Difficulties remain in making the correct diagnosis and developing a successful treatment plan for AD in human and veterinary medicine. The definition of AD itself is unclear and has been evolving for the last 20 y.26 In the absence of pathognomonic clinical signs and definitive diagnostic tests, the diagnosis of AD is mainly one of exclusion. One parameter, high IgE serum levels, has been used extensively but is considered insufficient in many cases, because AD in association with a normal level of serum IgE has been diagnosed.26 Currently, human and veterinary dermatologists rely on major and minor criteria for the diagnosis of AD, and only a few studies have proven reliable for diagnosis of AD in humans3,5,10,31 and dogs.10,30,34 The current case demonstrated several criteria consistent with the diagnosis of AD: maculopapular lesion, edema, erythema, pruritus, lesion located on glabrous skin, and a biopsy showing the presence of eosinophils and mast cells. Veterinarians caring for nonhuman primates might benefit from using similar diagnostic criteria derived from the human medical literature.
In conclusion, topical tacrolimus is a novel treatment option for AD in rhesus macaques. Tacrolimus may prove effective in other species that are affected by AD (for example, dogs8) or for other conditions (for example, feline asthma). To our knowledge, this article is the first report of an effective long-term treatment of AD by topical tacrolimus in a rhesus macaque.
References
- 1.Arkwright PD, Gillespie MC, Ewing CI, David TJ. 2007. Blinded side-to-side comparison of topical corticosteroid and tacrolimus ointment in children with moderate to severe atopic dermatitis. Clin Exp Dermatol 32:145–147 [DOI] [PubMed] [Google Scholar]
- 2.Astellas Pharma US. 2006 Patient information sheet: Protopic 0.1% (tacrolimus), NDC 0469-5202-30. [Google Scholar]
- 3.Brenninkmeijer EE, Schram ME, Leeflang MM, Bos JD, Spuls PI. 2008. Diagnostic criteria for atopic dermatitis: a systematic review. Br J Dermatol 158:754–765 [DOI] [PubMed] [Google Scholar]
- 4.Butler JM, Peters JE, Hirshman CA, White CR, Jr, Margolin LB, Hanifin JM. 1983. Pruritic dermatitis in asthmatic basenji–greyhound dogs: a model for human atopic dermatitis. J Am Acad Dermatol 8:33–38 [DOI] [PubMed] [Google Scholar]
- 5.De D, Kanwar AJ, Handa S. 2006. Comparative efficacy of Hanifin and Rajka's criteria and the UK Working Party's diagnostic criteria in diagnosis of atopic dermatitis in a hospital setting in North India. J Eur Acad Dermatol Venereol 20:853–859 [DOI] [PubMed] [Google Scholar]
- 6.Draelos ZD. 2008. Use of topical corticosteroids and topical calcineurin inhibitors for the treatment of atopic dermatitis in thin and sensitive skin areas. Curr Med Res Opin 24:985–994 [DOI] [PubMed] [Google Scholar]
- 7.Eichenfield LF, Hanifin JM, Beck LA, Lemanske RF, Jr, Sampson HA, Weiss ST, Leung DY. 2003. Atopic dermatitis and asthma: parallels in the evolution of treatment. Pediatrics 111:608–616 [DOI] [PubMed] [Google Scholar]
- 8.Gross TL, Ihrke PJ, Walder EJ, Affolter VK. 2005. Atopic dermtitis, p 200-206. Skin diseases of the dog and cat, 2nd ed Hoboken (NJ): Wiley-Blackwell [Google Scholar]
- 9.Hanifin JM. 2003. An overview of atopic dermatitis. Dermatol Nurs Suppl:6–9 [PubMed] [Google Scholar]
- 10.Hanifin JM, Rajka G. 1980. Diagnostic features of atopic dermatitis. Acta Derm Venereol Suppl (Stockh) 92:44–47 [Google Scholar]
- 11.Ingordo V, D'Andria G, D'Andria C. 2003. Adult-onset atopic dermatitis in a patch test population. Dermatology 206:197–203 [DOI] [PubMed] [Google Scholar]
- 12.Kulthanan K, Samutrapong P, Jiamton S, Tuchinda P. 2007. Adult-onset atopic dermatitis: a cross-sectional study of natural history and clinical manifestation. Asian Pac J Allergy Immunol 25:207–214 [PubMed] [Google Scholar]
- 13.Lerche CM, Philipsen PA, Poulsen T, Wulf HC. 2008. Topical tacrolimus in combination with simulated solar radiation does not enhance photocarcinogenesis in hairless mice. Exp Dermatol 17:57–62 [DOI] [PubMed] [Google Scholar]
- 14.Lubach D, Bensmann A, Bornemann U. 1989. Steroid-induced dermal atrophy. Investigations on discontinuous application. Dermatologica 179:67–72 [PubMed] [Google Scholar]
- 15.Macy JD, Jr, Huether MJ, Beattie TA, Findlay HA, Zeiss C. 2001. Latex sensitivity in a macaque (Macaca mulatta). Comp Med 51:467–472 [PubMed] [Google Scholar]
- 16.Morris J, Etheridge M. 2008. A case of suspected contact dermatitis in a juvenile cynomolgus monkey (Macaca fascicularis). J Med Primatol 37Suppl 1:56–59 [DOI] [PubMed] [Google Scholar]
- 17.Neumann E, Amtage D, Bruckner-Tuderman L, Mockenhaupt M. 2008. A single-center open-label long-term comparison of tacrolimus ointment and topical corticosteroids for treatment of atopic dermatitis. J Dtsch Dermatol Ges 6:548–553 [DOI] [PubMed] [Google Scholar]
- 18.Ovadia S, Wilson SR, Zeiss CJ. 2005. Successful cyclosporine treatment for atopic dermatitis in a rhesus macaque (Macaca mulatta). Comp Med 55:192–196 [PubMed] [Google Scholar]
- 19.Patel TS, Greer SC, Skinner RB., Jr 2007. Cancer concerns with topical immunomodulators in atopic dermatitis: overview of data and recommendations to clinicians. Am J Clin Dermatol 8:189–194 [DOI] [PubMed] [Google Scholar]
- 20.Patterson R, Harris K. 1981. Chronic pruritic dermatitis in asthmatic monkeys: a subhuman primate analogue of atopic dermatitis? Int Arch Allergy Appl Immunol 64:332–337 [DOI] [PubMed] [Google Scholar]
- 21.Rallis E, Korfitis C, Gregoriou S, Rigopoulos D. 2007. Assigning new roles to topical tacrolimus. Expert Opin Investig Drugs 16:1267–1276 [DOI] [PubMed] [Google Scholar]
- 22.Ring J, Mohrenschlager M, Henkel V. 2008. The US FDA ‘black box’ warning for topical calcineurin inhibitors: an ongoing controversy. Drug Saf 31:185–198 [DOI] [PubMed] [Google Scholar]
- 23.Russell JJ. 2002. Topical tacrolimus: a new therapy for atopic dermatitis. Am Fam Physician 66:1899–1902 [PubMed] [Google Scholar]
- 24.Rustin MH. 2007. The safety of tacrolimus ointment for the treatment of atopic dermatitis: a review. Br J Dermatol 157:861–873 [DOI] [PubMed] [Google Scholar]
- 25.Sehgal VN, Srivastava G, Dogra S. 2008. Tacrolimus in dermatology-pharmacokinetics, mechanism of action, drug interactions, dosages, and side effects: part I. Skinmed 7:27–30 [DOI] [PubMed] [Google Scholar]
- 26.Simpson EL, Hanifin JM. 2005. Atopic dermatitis. J Am Acad Dermatol 53:115–128 [DOI] [PubMed] [Google Scholar]
- 27.Singalavanija S, Noppakun N, Limpongsanuruk W, Wisuthsarewong W, Aunhachoke K, Chunharas A, Wananukul S, Akaraphanth R. 2006. Efficacy and safety of tacrolimus ointment in pediatric patients with moderate to severe atopic dermatitis. J Med Assoc Thai 89:1915–1922 [PubMed] [Google Scholar]
- 28.Stepkowski SM. 2000. Molecular targets for existing and novel immunosuppressive drugs. Expert Rev Mol Med 2: 1-23. Figure 2, Mechanism of action of cyclosporine or tacrolimus [DOI] [PubMed] [Google Scholar]
- 29.Virtanen H, Remitz A, Malmberg P, Rytila P, Metso T, Haahtela T, Reitamo S. 2007. Topical tacrolimus in the treatment of atopic dermatitis—does it benefit the airways? A 4-year open follow-up. J Allergy Clin Immunol 120:1464–1466 [DOI] [PubMed] [Google Scholar]
- 30.Willemse T. 1988. [Atopic dermatitis in dogs: current diagnostic criteria]Tijdschr Diergeneeskd 113:74–79 [Article in Dutch] [PubMed] [Google Scholar]
- 31.Williams HC, Burney PG, Hay RJ, Archer CB, Shipley MJ, Hunter JJ, Bingham EA, Finlay AY, Pembroke AC, Graham-Brown RA, Atherton DA, Lewis-Jones MS, Holden CA, Harper JI, Champion RH, Poyner TF, Launer J, David TJ. 1994. The UK Working Party's diagnostic criteria for atopic dermatitis. I. Derivation of a minimum set of discriminations for atopic dermatitis. Br J Dermatol 131:383–396 [DOI] [PubMed] [Google Scholar]
- 32.Winkelmann RK. 1986. Nicotinate white response of monkey sexual skin—a model for atopic reactivity. Clin Exp Dermatol 11:641–642 [DOI] [PubMed] [Google Scholar]
- 33.Wollenberg A, Reitamo S, Atzori F, Lahfa M, Ruzicka T, Healy E, Giannetti A, Bieber T, Vyas J, Deleuran M; European Tacrolimus Ointment Study Group.2008. Proactive treatment of atopic dermatitis in adults with 0.1% tacrolimus ointment. Allergy 63:742–750 [DOI] [PubMed] [Google Scholar]
- 34.Yager JA, Wilcox BP. 1994. Color atlas and text of surgical pathology of the dog and cat: dermatopathology and skin tumors, vol 1, p 62–63. London (UK): Wolfe Publishing [Google Scholar]