Abstract
Objective
Recent cadaver research demonstrates the perineal membrane’s ventral and dorsal portions and close relationship to the levator ani muscle. This study seeks to show these relationships in women by magnetic resonance (MR) images.
Methods
The subjects were 20 asymptomatic nulliparous women with normal pelvic examinations. MR images were acquired in multiple planes. Anatomical relationships from cadaver studies were examined in these planes.
Results
In the coronal plane the ventral perineal membrane forms an interconnected complex with the compressor urethrae, vestibular bulb and levator ani. The dorsal part connects the levator ani and vaginal side wall via a distinct band to the ischiopubic ramus. In the sagittal plane the parallel position of perineal membrane and levator ani are seen.
Conclusion
The perineal membrane’s anatomical features can be seen in women with MR. The close relationship between the perineal membrane and levator ani is evident.
Keywords: Perineal membrane, pelvic organ support, levator ani, MR imaging, pelvic floor
Introduction
Pelvic organ prolapse and stress urinary incontinence are distressing and common conditions.1–3 They are, in large part, caused by structural abnormalities in the muscles, ligaments and nerves of the pelvic floor.4,5 Modern cross sectional imaging has recently demonstrated its ability to demonstrate specific defects present in the pelvic organ support system to determine the precise anatomical problem in individual women, especially in the case of the levator ani muscle.6,7
The perineal membrane is an often discussed, yet rarely studied pelvic organ support structure. Formerly known as the urogenital diaphragm, it spans the triangle between the anterior portions of the urogenital triangle and attaches the pelvic organs laterally to the bony outlet.8,9 We have recently completed a study of the anatomy of this region as seen in cross sections of cadavers and dissection.9 This study revealed perineal membrane in women to be a composite structure with two unequal regions, 1) the ventral or anterior component where the membrane is formed by the blending and fusion of tissue from adjacent structures and 2) the more dorsal or posterior part which is a distinct fibrous sheet.
Previously this structure could only be evaluated in cadavers, but the advent of high resolution magnetic resonance (MR) imaging has allowed us to see the detailed nature of this important structure. There will be obvious importance to comparing this structure in women with and without pelvic floor disorders. This study seeks to evaluate MR imaging’s ability to visualize these relationships in living women and to describe which structural relationships are visible with this technique.
Methods
MR images from 20 asymptomatic, nulliparous women were examined. All subjects (aged 23 to 55 years) denied incontinence or prolapse symptoms and had full pelvic organ quantification (POP-Q) examinations confirming normal support. The women had been recruited as controls in case-control studies with MR for evaluation of prolapse and incontinence. All were asymptomatic, had not had prior pelvic floor surgery and had demonstrably normal support and continence on pelvic floor evaluation. Scans were performed on a 1.5 Tesla superconducting magnet (Signa; General Electric Medical Systems, Milwaukee, WI). Slice thickness was 4 mm with a gap of 1 mm, yielding 5 mm image spacing. A 160 × 160 mm field of view and 256 × 256 imaging matrix were used (1.5 T Signa, GE medical systems). Supplemental images were made on a 3T scanner (Achieva, Philips Medical Systems using an 8 channel phased-array cardiac coil. Proton density sequences (TR 2107, TE 30) where obtained (4mm slice, 1mm gap, 256×256 matrix, NSA 2) in the coronal, axial and sagittal planes through the pelvis. Additional higher resolution (2mm slice 0.2 gap, 256×256 matrix, NSA 2) proton density sequences (TR 2107, TE 30) and proton density with fat saturation sequences (TR 2355, TE 30) were obtained in the axial and coronal planes of the anterior (ventral) pelvic floor in selected women. The imaging planes were angled in both the axial and coronal planes to better demonstrate the anatomy when necessary.
When plane adjustment was needed the axial plane was along the pelvic floor from the inferior pubic symphysis to the external anus. The coronal plane was angled midway between the standard coronal and an orthogonal plane from the study axial plane. The sagittal was a straight sagittal plane through the midline. The location of known attachment points, structural connections and anatomical relationships from previous cadaver studies were sought and qualitatively evaluated in each plane. The authors reviewed the images together and agreed on the planes best able to demonstrate the relationships seen at the cadaver studies.
A 3-D model was generated to better visualize the anatomical relationships of the perineal membrane utilizing a subject’s original axial, sagittal and coronal DICOM (Digital Imaging and Communications in Medicine) images. Using 3-D Slicer imaging software (v 2.1b1, Brigham and Women’s Hospital, Boston, MA), the static images were aligned using bony landmarks. Anatomical structures bounding the perineal membrane were outlined in the best-visualized plane. These tracings were then combined to create the 3-D model. Structure tracings and 3-D models were reviewed by the senior author and accuracy assured by confirming the fidelity of the model to previous experience with dissection and cross sectional anatomical studies.
Results
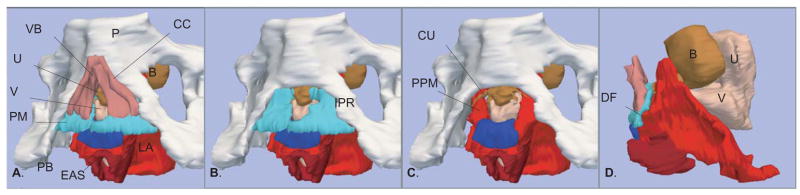
The general anatomical relationships of the perineal membrane can be appreciated from an overview as provided on the 3D reconstructions from a 25 year old nulliparous woman (Figure 1). The perineal membrane forms a sloping triangular plane from the narrow ventral or anterior attachment on the pubic bones to the dorsal or inferior posterior border on the ischiopubic ramus at the level of the perineal body. In the reconstruction the layered relationship to the erectile tissues of the vestibular bulb and the clitoral crus can be seen (Figure 1, A and B). The more complex blended features of ventral or anterior perineal membrane to the clustered structures around the urethra are not as apparent (Figure 1, C). The nearly parallel course of the perineal membrane and the adjacent pubic portion of the levator ani muscle can be seen to intersect near the vaginal side wall where the muscle fuses with the lateral vaginal wall and the superior or cranial surface of the perineal membrane (Figure 1, D).
Figure 1.
The three-dimensional relationships are best seen in the models generated from MR images of a 27 year old nullipara. A. Oblique left inferolateral view showing structures related to perineal membrane. B. Same view with clitoral crus and vestibular bulb removed to show the perineal membrane cephalad to these structures, extending bilaterally to the ischiopubic rami. C. Same view with the perineal membrane removed to illustrate its relation to the compressor urethra and anterior portion of the levator ani. D. Left lateral supine view of structures with pubic bone removed to illustrate parallel nature of levator ani to the ventral perineal membrane and the dorsal fusion (DF) of these structures. Pubic bone (PB) – white; Clitoral Crus (CC) and Vestibular Bulb (VB) – dark pink; Urethra (U), Compressor Urethra (CU), and Bladder (B)– brown; Vagina (V)– light pink; Perineal Membrane (PM) – turquoise; Puboperineal Muscle (PPM); Perineal Body (PB) – dark blue; External Anal Sphincter (EAS) – dark red; Levator Ani (LA) – red. ©DeLancey 2008
Anterior or Ventral Component
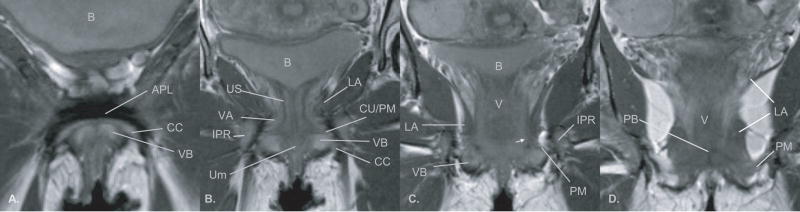
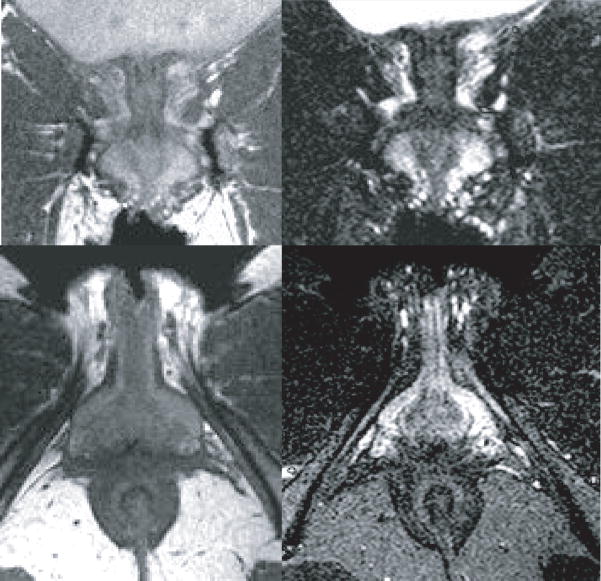
The anterior pelvic floor has complex anatomy because of the number of structures in this small confined space. Certain portions of this anatomy are better seen in one plane as opposed to another because of the orientation of the tissue or because there is more MR tissue contrast. In general the coronal plane describes the ventral or anterior relations of the perineal membrane. The posterior capsule of the pubic symphysis and the bilateral pubic bodies are connected with the arcuate pubic ligament (Figure 2, A). At the inferior surface of the pubic symphysis the paraurethral connective tissue blends with some of these dense fibers as the urethra bends around the pubic symphysis. Slightly more superior this symphysial fibrous complex provides the thin bands of the arcus tendineus fascia pelvis. These fibers are continuous with the fascia of the anterior levator ani muscles and they blend into the mass of tissue which forms the anterior or ventral portion of the perineal membrane (Figure 2, B). The arcus tendineus fascia pelvis fuses with paraurethral and paravaginal connective tissues which contain the compressor urethrae and the urethrovaginal sphincter muscles of the distal urethra (Figure 2, B). (N.B. These muscles were formerly known as the deep transverse perineal muscles). Here the prominent vascularity of the area can be seen on MR comparing standard imaging sequence with fluid sensitive sequence in the same coronal slice plane to differentiation vessels from fat. (Figure 3, A and B). In these sequences, because of the techniques used to create the image, the MR signal is suppressed from fat so it is no longer bright and the signal is enhanced from fluid so that vasculature and fluid stand out against the dark background. The extensive vascular supply of the vestibular bulbs and the clitoral crus are clearly demonstrated in the axial view as is the size and complexity of the paraurethral vessels (Figure 3, C and D). These images also show that the perineal membrane is separated from the levator ani by this network of paraurethral and paravaginal vessel anastomosis.
Figure 2.
Proton density MR image in the coronal plane of a nullipara showing anatomic relationships from the level of the arcuate pubic ligament (APL) ventrally (panel A) to the perineal body (panel D) dorsally. Abbreviations: bladder (B), clitoral crus (CC), vestibular bulbs (VB), arcuate pubic ligament (APL), striated urogenital sphincter muscle (US), external urethral meatus (Um), perineal membrane (PM), compressor urethra (CU), ischiopubic ramus (ISR), levator ani muscles (LA), vascular anastomosis between the pudendal and periurethral vessels (VA), vagina (V), perineal membrane (arrow head), perineal body (PB). ©DeLancey 2008
Figure 3.
Coronal (top) and axial (bottom) plane comparing standard proton density sequence (left) to fluid sensitive proton density with fat saturation (right) which enhances the bright signal from the vasculature. (The coronal plane is comparable to that in Figure 1 panel B and the axial plane is comparable that in Figure 5, A). ©DeLancey 2008
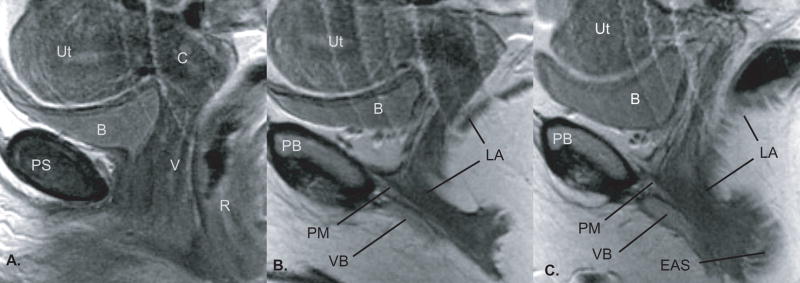
The inferior surface of the perineal membrane is intimately related to the anterior levator ani muscles which fuse with the paraurethral and paravaginal connective tissues. The inferior surface of the perineal membrane is fused with the superior or cranial margins of the vestibular bulb and the clitoral crus with their investing muscle fibers. These relationships can be seen in the sagittal plane (Figure 4, B and C). In this anterior region the perineal membrane is not a discrete or well defined isolated structure but rather a plane of coalescing tissue defined by these relationships (Figure 2, B).
Figure 4.
Proton density images in the sagittal plane, same individual shown in Figure 2 (non-contiguous) from the midline (Panel A) toward the lateral vaginal wall (Panel C). The anatomy as seen in the sagittal plane reveals the course of the perineal membrane from the pubic bone posterior cortex obliquely downward toward the region of the perineal body. Abbreviations: pubic symphysis (PS), bladder (B), uterus (Ut), cervix (C), vagina (V), rectum (R), perineal membrane (PM), pubic bone (PB), levator ani muscles (LA), vestibular bulbs (VB), external anal sphincter (EAS). ©DeLancey 2008
Dorsal or Posterior Portion
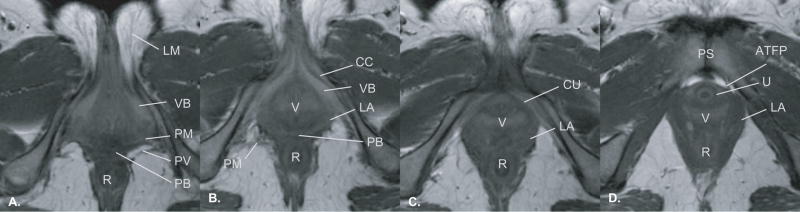
At about the level of the vaginal lumen, the perineal membrane begins to take on a separate appearance as a single fibrous band extending laterally to the inferior pubic ramus (Figure 2, C and D). The connective fibers within the membrane run in a transverse direction between the midline to the lateral wall. The fascia of the levator ani muscles continue to be fused along the superior surface of the membrane but the percentage of the levator ani muscle’s contribution to the superior margin decreases as the membrane flares out to the widening angle of the ischiopubic ramus (Figure 2, D). At the perineal body, the perineal membrane clearly separates the superior ischiorectal fossa fat and the inferior perineal structures. The axial plane demonstrates the arching posterior border with the pudendal neurovascular bundle approaching the lateral attachment (Figure 5, A and B).
Figure 5.
Axial images from caudad, inferior, (Panel A) to cephalad, superior, (Panel D) showing anatomic relationships of the perineal membrane. Because the perineal membrane is slightly oblique to the axial plane, it is not seen well in this imaging plane. The surrounding structures (e.g. vestibular bulb, levator ani muscle) allow its location to be inferred even though a separate structure can not been seen. Abbreviations: labia majora (LM), rectum (R), vestibular bulbs (VB), perineal membrane (PM), pudendal vessels (PV), perineal body (PB), vagina (V), compressor urethrae (CU), arcus tendineus fascia pelvis (ATFP), pubic symphysis (PS), urethra (U), levator ani muscle (LA), urethra (U). ©DeLancey 2008
Discussion
This study demonstrates that the perineal membrane and its structural relationships can be seen in MR images. The relationships with the vestibular bulb, clitoral crus, and levator ani muscles are clearly visible as is the lateral attachment to the ischiopubic ramus. The differences between the ventral portion where it lies within a solid mass of adjacent tissues and the dorsal part where it is a freer standing sheet of tissue are easily demonstrated. The coronal and sagittal scans are the most helpful with seeing these relationships, but the axial scan does show some of these features as well. It demonstrates in living women the relationships that have been seen in cadavers and avoids the distortion normally associated with the embalming process.
There are a number of structure function relationships that these findings suggest. The posterior or dorsal portion of the perineal membrane provides a broad fibrous connection among the midline soft tissues to the lateral bony support of the pelvis. The levator ani muscle fascia fuses with the superior surface and both attach to the vaginal side walls. The perineal membrane also blends with the perineal body which itself is a coalescence of midline dense fibers. With these multiple interconnections the perineal membrane could provide a component of support or restraint to the tissues acting like a retinaculum to help maintain alignment. Such thin bands of highly organized regular connective tissue are found elsewhere in the body where they change the angle of muscle pull by restricting motion. The direction of their internal fiber bundles is related to the mechanical stress they normally experience. These predominately collagen fibers also have interwoven fibers of collagen and elastin which increase strength to resist shear forces and provide some flexibility. Common examples are the flexor and extensor retinaculum of the wrist, the fine cruciate and pulley ligaments of the fingers or the flexor retinaculum of the tarsal tunnel. Other structures like the plantar or palmer aponeurosis spread multiple muscles forces across a wide area of distant structures by a connecting sheet of tough fibers. Because these structures can be so small that they are easily overlooked, their function may not be readily appreciated in the normal state. However, when damaged, their role in maintaining normal motion becomes apparent as the power of the muscles they usually constrain or channel becomes misdirected or weakened.
Vaginal birth can cause extensive damage to perineal tissues including the levator ani muscles.10 Damage to the perineal membrane or its closely related component parts like the levator ani muscle may result in loss of normal spatial relationships and possible weakening of mechanical function and the ability to see these structures to scans should provide the ability to objectively study their damage. The adjacent levator ani muscle which is frequently injured during birth11 extends in an angle from its superior anterior and lateral wall attachments to its more inferior midline connections which it shares with the perineal membrane. Disruption of these structures would be expected to have functional consequences. The membrane is almost a flat plane connecting the midline to the side wall. Levator ani muscle contraction should provide balanced aligned force to pull the pelvic floor superior and anterior thereby narrowing the genital hiatus. In the absence of the perineal membrane’s constraining or anchoring position the muscle force may produce asymmetric or dysfunctional motion. While the perineal membrane’s possible constraining role is more readily appreciated in the band-like dorsal or posterior portion, because of its wide anterior interrelationships force could also be channeled among these structures. Though the perineal membrane’s association with the dense connective tissues around the pubic symphysis, the power of the levator ani muscles at the midline could be stabilized by the ventral portion where it coalescences with both the softer paraurethral and paravaginal tissues and the denser symphysial tissue. Therefore the perineal membrane should not be ignored as a passive structure buried in the pelvic floor. Because of its structure and its multiple interconnections the perineal membrane may perform a dynamic role in the function of the levator ani, a function which will probably be better seen as the relationships of the levator ani and the perineal membrane are examined in the altered pathological state of prolapse.
This was a retrospective examination of 20 normal nulliparous women who were participants in MR studies designed to look at other areas of anatomy. The perineal membrane and the associated structures are small and difficult to image in standard planes. Optimal imaging planes for the perineal membrane have yet to be determined. Better anatomic definition on MR imaging could be achieved with attention to hardware and software changes that improve spatial resolution. Protocol adjustments such as scanning with thinner slice thickness (2 mm instead of 4 mm) and using a higher matrix (512× 512 instead of256 × 256) also provide greater spatial resolution. However, even with these limitations, it has been possible to obtain excellent images of this area.
Specialized sequences such as the use of fat saturation or STIR can enhance soft tissue contrast and provide more information about structures which appear similar on more standard imaging sequences.
Advances in modern imaging have brought our abilities to specifically study birth related trauma and related disease. As was true when the structure of the anal sphincter could be seen with ultrasound, important advances in preventing and treating sphincter rupture followed.12 Similar advances should be possible as our ability to understand the appearance of pelvic organ support structures and their damage grows.
Acknowledgments
We gratefully acknowledge support from the Office for Research on Women’s Health SCOR on Sex and Gender Factors Affecting Women’s Health and the National Institute of Child Health and Human Development Grants 1 P50 HD044406 and R01 HD 38665
Footnotes
To be presented at the 29th Annual Scientific Meeting of the American Urogynecologic Society, Chicago, IL, September 4–6, 2008.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Olsen AL, Smith VJ, Bergstrom JO, Colling JC, Clark AL. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997;89:501–6. doi: 10.1016/S0029-7844(97)00058-6. [DOI] [PubMed] [Google Scholar]
- 2.Boyles SH, Weber AM, Meyn L. Procedures for pelvic organ prolapse in the United States, 1979–1997. Am J Obstet Gynecol. 2003;188:108–15. doi: 10.1067/mob.2003.101. [DOI] [PubMed] [Google Scholar]
- 3.Boyles SH, Weber AM, Meyn L. Procedures for urinary incontinence in the United States, 1979–1997. Am J Obstet Gynecol. 2003;189:70–5. doi: 10.1067/mob.2003.376. [DOI] [PubMed] [Google Scholar]
- 4.Smith AR, Hosker GL, Warrell DW. The role of pudendal nerve damage in the aetiology of genuine stress incontinence in women. Br J Obstet Gynaecol. 1989;96(1):29–32. doi: 10.1111/j.1471-0528.1989.tb01572.x. [DOI] [PubMed] [Google Scholar]
- 5.DeLancey JO, Morgan DM, Fenner DE, Kearney R, Guire K, Miller JM, Hussain H, Umek W, Hsu Y, Ashton-Miller JA. Comparison of levator ani muscle defects and function in women with and without pelvic organ prolapse. Obstet Gynecol. 2007;109(2 Pt 1):295–302. doi: 10.1097/01.AOG.0000250901.57095.ba. [DOI] [PubMed] [Google Scholar]
- 6.DeLancey JO, Kearney R, Chou Q, et al. The appearance of levator ani muscle abnormalities in magnetic resonance images after vaginal delivery. Obstet Gynecol. 2003;101:46–53. doi: 10.1016/s0029-7844(02)02465-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dietz HP. Lanzarone V Levator trauma after vaginal delivery. Obstet Gynecol. 2005;106:707–712. doi: 10.1097/01.AOG.0000178779.62181.01. [DOI] [PubMed] [Google Scholar]
- 8.Oelrich TM. The striated urogenital sphincter muscle in the female. Anat Rec. 1983;205:223–32. doi: 10.1002/ar.1092050213. [DOI] [PubMed] [Google Scholar]
- 9.Stein TA, DeLancey JOL. Structure of the perineal membrane in females: gross and microscopic anatomy. Obstet Gynecol. 2008;111(3):686–93. doi: 10.1097/AOG.0b013e318163a9a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dietz HP, Gillespie AV, Phadke P. Avulsion of the pubovisceral muscle associated with large vaginal tear after normal vaginal delivery at term. Aust N Z J Obstet Gynaecol. 2007;47(4):341–4. doi: 10.1111/j.1479-828X.2007.00748.x. [DOI] [PubMed] [Google Scholar]
- 11.DeLancey JO, Kearney R, Chou Q, et al. The appearance of levator ani muscle abnormalities in magnetic resonance images after vaginal delivery. Obstet Gynecol. 2003;101:46–53. doi: 10.1016/s0029-7844(02)02465-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sultan AH, Kamm MA, Hudson CN, Thomas JM, Bartram CI. Anal-sphincter disruption during vaginal delivery. N Engl J Med. 1993;23;329(26):1905–11. doi: 10.1056/NEJM199312233292601. [DOI] [PubMed] [Google Scholar]