Abstract
The genetically epilepsy-prone rat (GEPR) exhibits inherited predisposition to sound stimuli-induced generalized tonic-clonic seizures (audiogenic reflex seizures) and is a valid model to study the physiopathology of epilepsy. In this model, the inferior colliculus (IC) exhibits enhanced neuronal firing that is critical in the initiation of reflex audiogenic seizures. The mechanisms underlying IC neuronal hyperexcitability that leads to seizure susceptibility are not as yet fully understood. The present report shows that the levels of protein expression of SK1 and SK3 subtypes of the small conductance Ca2+-activated K+ channels were significantly decreased, while SK2 channel proteins were increased in IC neurons of seizure-naive GEPR-3s (SN-GEPR-3), as compared to control Sprague-Dawley rats. No significant change was found in the expression of BK channel proteins in IC neurons of SN-GEPR-3s. Single episode of reflex audiogenic seizures in the GEPR-3s did not significantly alter the protein expression of SK1-3 and BK channels in IC neurons compared to SN-GEPR-3s. Thus, downregulation of SK1 and SK3 channels and upregulation of SK2 channels provide direct evidence that these Ca2+-activated K+ channels play important roles in IC neuronal hyperexcitability that leads to inherited seizure susceptibility in the GEPR.
Keywords: Seizure susceptibility, Calcium-activated potassium channel, Protein, Inferior colliculus
1. Introduction
Defective ion channels are thought to play important roles in the mechanisms underlying neuronal hyperexcitability that leads to inherited epilepsies in human (Reid et al. 2009). The genetically epilepsy-prone rat (GEPR) exhibits inherited susceptibility to generalized tonic-clonic seizures elicited by auditory stimulus (reflex audiogenic seizures) and is a relevant model to study the physiopathology of generalized seizures and epilepsy (Faingold 1999). In this model, the inferior colliculus (IC) exhibits an enhanced neuronal firing that is critical in the initiation of reflex audiogenic seizures (Faingold 1999). Two sub-strains of the GEPR have been characterized including the GEPR-3 that exhibits generalized clonic seizures, while the GEPR-9 displays generalized tonic-clonic seizures following exposure to the same sound stimulus (Dailey et al. 1989). Evidence suggests that both GEPR-3 and GEPR-9 exhibit similar pharmacological sensitivity suggestive of similar mechanisms, at least, in the initiation of reflex audiogenic seizures (Dailey and Jobe 1985). Although ion channels play an important role in the control of IC neuronal excitability, their involvement in the development of inherited seizure susceptibility in the GEPR is not yet fully understood (Evans et al. 1994, 2006; Verma-Ahuja et al. 1995; Molnar et al. 2000; Faingold 2002). We have recently reported that the current density of high threshold voltage-gated Ca2+ channels was markedly enhanced in IC neurons of the GEPR-3 (N’Gouemo et al. 2009). Such increase of Ca2+ currents is suggestive of a massive Ca2+ influx resulting in abnormal levels of cytosolic Ca2+ and disturbance of Ca2+ homeostasis, which in turn can alter Ca2+-induced Ca2+ release from intracellular stores and deregulated Ca2+-dependent mechanisms including K+ channels. Ca2+-activated K+ channels are classified into large (BK), intermediate (IK), and small (SK) conductance channels on the basis of their electrophysiological, pharmacological and molecular profiles (Köhler et al. 1996; Sah 1996; Stocker and Pedarzani 2000). SK subtype of Ca2+-activated K+ channels are encoded by three different genes (SK1, SK2, and SK3) resulting in distinct channels, which are expressed in many brain loci including the IC (Köhler et al. 1996; Stocker and Pedarzani 2000). Evidence suggests that BK channels play important roles in the induction of fast repolarization and afterhyperpolarization (AHP) associated with the action potential and bursting, while SK1 channels as well as SK2 and SK3 channels contribute to the slow AHP and medium AHP, respectively (Sah 1996; Pedarzani et al. 2001). BK channels are activated by both voltage and in increases in intracellular Ca2+, while SK channels are exclusively activated by submicromolar increases in intracellular Ca2+ interacting with a cytosolic calmodulin-binding domain of the channel (Xia et al. 1998). Electrophysiological studies reported that IC neurons expressed both BK and SK channels and these Ca2+-activated K+ conductances exhibited both fast and slower AHP (Li et al. 1998; Sivaramakrishnan and Oliver 2001). Since Ca2+-activated K+ channels are thought to play important roles in control of neuronal excitability, altered expression of these channels in the IC may contribute to reflex audiogenic seizure susceptibility in the GEPR. Interestingly, the amplitude of both slow and fast AHP potentials was reduced in hippocampus CA3 neurons of the GEPR and in human epilepsy suggesting a downregulation of these channels (Verma-Ahuja et al. 1995; Williamson et al. 1993). In addition, pharmacological studies reported that blockade of apamin-sensitive channels within the IC elicited spontaneous seizures and decreased the threshold for electrically-induced seizures from the IC in normal rats; these findings therefore highlighted the role of apamin-sensitive SK channels in generation of non-audiogenic seizures (McCown and Breese 1990). Abnormal function of BK channels also has been implicated in the mechanisms of seizures and genetic epilepsy (Brenner et al. 2005; Lorenz et al. 2007; Ermolinsky B., Arshadmansab et al. 2008, Du et al., 2005; Shruti et al. 2008; Pacheco et al. 2008). Here, we sought to determine the levels of protein expression of BK, SK1, SK2, and SK3 subtypes of Ca2+-activated K+ channels in IC neurons obtained from normal Sprague-Dawley rats and quantify their changes in seizure-naive GEPR-3 (SN-GEPR-3) and seizure-experienced GEPR-3 (SE-GEPR-3). A preliminary report of these studies has already appeared (N’Gouemo et al., 2003).
2. Results
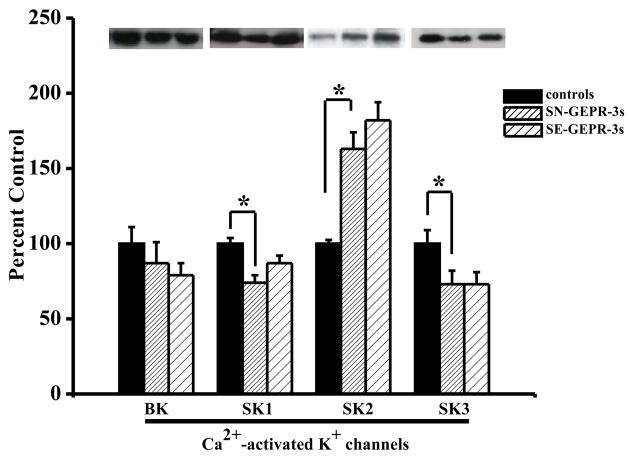
Each GEPR-3 tested for seizure susceptibility only exhibited one episode of reflex audiogenic clonic seizures. SN-GEPR-3 and control Sprague-Dawley rats were not tested and did not exhibit seizures. The presence of Ca2+ -activated K+ channel protein in IC neurons was determined by using rabbit polyclonal antibodies to BK, SK1, SK2 and SK3 subtypes of Ca2+-activated K+ channels. BK channels were identified as an immunoreactive band with a molecular mass of 125 kDa while SK1, SK2 and SK3 channels were observed at 75 kDa, 60 kDa and 80 kDa, respectively. Western blot analysis showed that IC neurons expressed all subtypes of Ca2+ -activated K+ channels (Figure 1). Quantification shows that levels of protein expression of SK1 subtype of Ca2+ -activated K+ channels were significantly (F = 7.3, P = 0.004) decreased in IC neurons of SN-GEPR-3, as compared to control SD rats (Figure 1). The levels of protein expression of SK3 subtype of Ca2+ -activated K+ channels also were significantly (F = 10.8, P = 0.001) decreased in IC neurons of SN-GEPR-3 (n = 8), as compared to control SD rats (n = 8) (Figure 1). In contrast to SK1 and SK3 channels, the levels of protein expression of SK2 subtype of Ca2+ -activated K+ channels were significantly (F = 5.3; P = 0.01) increased in IC neurons of SN-GEPR-3 (n = 8), as compared to control SD rats (n = 8) (Figure 1). No change in the levels of protein expression of SK1-3 channels was found in SE-GEPR-3 (n=8) compared to SN-GEPR-3 (n=8) (Figure 1); in fact, seizure episodes appear to non-significantly restore the levels of protein expression of SK1 channels to nearly control levels. Seizure episodes also non-significantly increased the levels of protein expression of SK2 channels. The data also shows that the levels of protein expression of BK subtype of Ca2+-activated K+ channels were non-significantly (F = 0.2; P = 0.8) decreased in IC neurons of SN-GEPR-3 (n = 8) as compared to control SD rats (n = 8), as well as in SE-GEPR-3 (n = 8) compared to SN-GEPR-3 (n = 8).
Figure 1.
Quantification of the protein expression of Ca2+-activated K+ channels. Representative immunoblots of BK, SK1, SK2 and SK3 subtypes of Ca2+-activated K+ channels obtained from IC neurons of control SD rats, SN-GEPR-3s and SE-GEPR-3s are showed in insets. The levels of protein expression of SK1 and SK3 subtypes of Ca2+-activated K+ channels were significantly decreased in IC neurons of SN-GEPR-3s compared to control SD rats. In contrast, the levels of proteins associated with SK2 channels were significantly increased in IC neurons SN-GEPR-3s compared to control SD rats. Seizure episodes slightly increased the protein expression of SK1 and SK2 channels in IC neurons compared to SN-GEPR-3s, but did not alter expression of SK3 channels. The levels of protein expression of BK channels were slightly reduced in SN-GEPR-3s compared to control SD rats as well as in SE-GEPR-3s compared to SN-GEPR-3s. Each column represents the mean ± S.E.M. (n = 8). *P <0.05 (ANOVA followed by post hoc test).
3. Discussion
The main finding of this study is that levels of protein expression associated with SK1 and SK3 subtypes of Ca2+ -activated K+ channels are significantly reduced in IC neurons of SN-GEPR-3. In contrast, the levels of protein expression of SK2 channels are significantly increased, while the levels of BK channels are not significantly altered in IC neurons of SN-GEPR-3. Seizure episodes do not significantly alter the expression of SK1-3 and BK subtypes of Ca2+ -activated K+ channels. These findings indicate that downregulation of SK1 and SK3 channels as well as upregulation of SK2 channels in IC neurons are seizure susceptibility-related phenomena in the GEPR-3.
Multiple lines of evidence indicated that IC neurons are critical in the initiation of reflex audiogenic seizures in the GEPR-9 (Browning 1986; Faingold 1999). At the transition to seizures, unlike other brain sites, the IC exhibits sustained increases in neuronal firing, which support the role of this brain site in the initiation of reflex audiogenic seizures (Faingold 1999; N’Gouemo et al 1998). Such changes in IC neuronal firing may also occur in the GEPR-3. In addition to audiogenic seizures, both GEPR-9 and GEPR-3 also exhibited enhanced propensity to seizures induced by non-audiogenic stimuli including limbic kindling (Savage et al. 1986). In line with this report, functional ion channel abnormalities were reported in the hippocampus of the GEPR-9, a brain site that is not implicated in the initiation of reflex audiogenic seizures (Evans et al. 1994, Verma-Ahuja and Pencek, 1994; Verma-Ahuja et al. 1995). The mechanisms underlying the increases in IC neuronal firing and inherited seizure susceptibility in the GEPR-9 and GEPR-3 are not as yet well understood. The large (BK) and small (SK) conductance, Ca2+ -activated K+ channels are known to play important roles in the control of IC neuronal excitability, and altered expression of these channels therefore may play important roles in seizure susceptibility and seizure generation (Li et al 1998; Sivaramakrishnan and Oliver 2001). In support of this idea, electrophysiological study reported that the amplitude of slow AHP was smaller in hippocampal neurons of the GEPR-9 (Verma-Ahuja and Pencek, 1994; Verma-Ahuja et al. 1995; 1998). Such altered slow AHP also was found in human with epilepsy suggesting that abnormal AHP may be a general mechanism underlying neuronal hyperexcitability across species (Williamson et al. 1993). Ca2+ -activated K+ channels also may play an important role in the generation of non-audiogenic seizures, since pharmacological blockade of apamin-sensitive Ca2+ -activated K+ channels, including SK2 and SK3 channels, within the IC triggered spontaneous seizures and electrically-evoked seizures in normal rats (McCown and Breese 1990). Whether the blockade of Ca2+ -activated K+ channels within the IC induces reflex audiogenic seizure susceptibility in normal rats remains unknown. Nevertheless, the levels of protein expression of SK1 and SK3 channels were downregulated in IC neurons of the GEPR-3 suggesting that these channels may play an important role in inherited audiogenic seizure susceptibility. BK subtype of Ca2+-activated K+ channels also may play an important role in neuronal hyperexcitability that leads to seizures. Indeed, the amplitude of fast AHP associated with BK channels was smaller in hippocampal neurons of the GEPR-9 suggesting that down regulation of these channels may contribute to neuronal hyperexcitability associated with non-audiogenic seizure susceptibility. In line with this idea, the expression of BK channel protein was only slightly reduced in IC neurons of the GEPR-3, indicative of a minor role of these channels in the mechanisms underlying audiogenic seizure susceptibility.
In contrast to SK1 and SK3 channels, the levels of protein expression of SK2 channels were significantly enhanced in IC neurons of the GEPR-3. These findings suggested that upregulation of SK2 channels may represent a compensatory mechanism to the downregulation of SK1 and SK3 channels in IC neurons. Alternatively, upregulation of SK1 channels may contribute to enhanced seizure susceptibility in the GEPR-3. In support of this concept, recent reports indicated that a gain-of-function of BK channels was associated with picrotoxin-induced seizures and inherited epilepsy, while blockade of these channels suppressed chemically-induced seizures (Brenner et al. 2005; Du et al. 2005; Shruti et al. 2008; Sheehan et al 2008).
We conclude that altered levels of expression of SK1-3 subtypes of Ca2+-activated K+ channels provide direct evidence that these channels contribute to IC neuronal hyperexcitability that leads to reflex audiogenic seizures in the GEPR. Functional studies are required to determine whether altered expression these channels is a critical step for seizure generation in the GEPR.
4. Experimental Procedure
Male GEPR-3 (8–12 week-old) were obtained from the colony maintained at Southern Illinois University, Springfield, IL. This animal strain derives from Sprague-Dawley rats by selective breeding for audiogenic seizure susceptibility. Age-matched Sprague-Dawley rats purchased from Taconic (MD) were used as controls. Spontaneous seizures are rare in the GEPR-3 and were not monitored in this study. Two groups of GEPR-3 were used: seizure naive GEPR-3 (SN-GEPR-3) refer to animals that have never been exposed to sound stimulus-induced seizures, while seizure exposed-GEPR-3 (SE-GEPR-3) refer to animals that have been exposed to loud sound stimulus-induced seizures. SN-GEPR-3 and SE-GEPR-3 were selected randomly from the colony. In order to elicit seizures, GEPR-3s were exposed to an electrical bell (120 dB SPL, re: 0.0002 dynes/cm2) until seizure onset or for a maximum of 60 sec. In this study, all SE-GEPR-3 exhibited one episode of generalized seizures consisted of wild running that evolved into clonic seizures. The severity of seizures was scored according to the scale for audiogenic seizures (Jobe et al. 1973). SE-GEPR-3 were used 4–6 weeks after seizure induction; this time interval avoids the effects of acoustic stimulation and acute effects of seizures themselves on the protein expression of Ca2+-activated K+ channels. All experimental procedures were approved by the Institution Animal Care and Use Committee, and all efforts were made to minimize the number of animals used and their suffering.
Animals were deeply anesthetized with pentobarbital (100 mg/kg; i.p.). The brains were removed and colliculi were quickly dissected (the periaqueductal grey was discarded) and stored at −70°C until processed. Tissue homogenates were prepared according to the method described by Twombly et al. (1979). Brielfy. IC homogenates from each animal were prepared in TE buffer (10 mM Tris-HCl, pH 7.4; 1 mM EDTA) and centrifuged at 30,000 X gmax for 16 min at 4°C. The crude membrane pellets were resuspended in TE buffer. Protein concentrations in the crude membrane suspensions were determined using Bicinchoninic acid (BCA) Assay Reagent (Pierce, Rockford, IL) and each sample was diluted to a protein concentration of 10 mg/ml in TE buffer. The optimal Ca2+-activated K+ channel concentration of 15 μg/lane used in this study was determined by testing several concentrations: 10, 15, and 30 μg of protein per lane. The membrane proteins were separated using sodium dodecyl sulfate-polyacrylamide gel (containing 7.5% polyacrylamide; Invitrogen, Carlsbad, CA) electrophoresis and transferred to the polyscreen polyvinylidene fluoride (New England Nuclear, Boston, MA) membranes in transfer buffer (25 mM Tris-HCl, 192 mM glycine, 20% methanol). The blots were processed using methods similar to those described by Towbin et al. (1979). Briefly, the blots were blocked in TBST buffer (20 mM Tris-HCl, pH 7.4; 140 mM NaCl and 0.1% v/v Tween 20; TBST) containing 5% nonfat dry milk (BLOTTO buffer) then incubated with Ca2+-activated K+ channel BK, SK1, SK2, and SK3 antibodies (Alomone labs, Jerusalem, Israel) at concentrations of 1–1.5 μg/ml, overnight at 4oC. The following day, the blots were washed with a TBST buffer and incubated with a donkey anti-rabbit antibody (Amersham, Arlington Heights, IL) at a dilution of 1:5000 in BLOTTO buffer for 30 min at room temperature. After several washes of the blots, individual bands were visualized on the Hyperfilm (Amersham, Piscataway, NJ) using the enhanced chemiluminescence Super Signal West Pico and/or Femto (Pierce, Rockford, IL).
To quantify the protein levels of each Ca2+-activated K+ subtype, the exposed films were analyzed and individual bands quantified by computer-assisted densitometry using a BioImage Analysis System (Ann Arbor, MI), which calculated the integrated intensity of a band using both the density and the area of the band. In each experiment, the integrated intensity of a specific protein band was compared to a standard curve of known protein concentration from rat hippocampus as done previously in our laboratory (N’Gouemo et al., 2006; Wang et al., 1995). The integrated intensities of the bands from experimental tissues determined at the same time and on the same piece of film were compared with the interpolated standard curve to yield a value of subunit expression relative to the standard hippocampus tissue. The utilized integrated intensities were always in the linear part of the standard curve. To determine differences in the protein expression between groups, one way analysis (ANOVA) was performed followed by Student-Newman-Keuls post hoc test. Data fulfill to the normality test (Shapiro-Wilk test) and tests for homogeneity of variance (Levene and Brown-Forsthe tests). Data obtained from SN-GEPR-3 were compared to control SD rats to determine changes associated with seizure susceptibility whereas findings in SE-GEPR-3s were compared to SN-GEPR-3s to assess the effects of seizure episodes. Data are presented as mean ± S.E.M.
Acknowledgments
This publication was made possible by Public Health Service grants (NS047193 to P.N, AA11284 to R.P.Y. and AA11628 to C.L.F.) from the National Institutes of Health (NIH) and its contents are responsibility of the authors and do not necessarily represent the official views of NIH.
Abbreviations
- AHP
afterhyperpolarization
- BK
large conductance, Ca2+-activated K+ channel
- GEPR
genetically epilepsy-prone rat
- IC
inferior colliculus
- IK
intermediate conductance, Ca2+-activated K+ channel
- SE-GEPR-3s
seizure-experienced GEPR-3s
- SK
small conductance, Ca2+-activated K+ channel
- SN-GEPR-3s
seizure-naive GEPR-3s
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature references
- Browning RA. Neuroanatomical localization of structures responsible for seizures in the GEPR: lesion studies. Life Sci. 1986;39:857–867. doi: 10.1016/0024-3205(86)90367-x. [DOI] [PubMed] [Google Scholar]
- Dailey JW, Jobe PC. Anticonvulsant drug and the genetically epilepsy-prone rat. Fed Proc. 1985;44:2640–2644. [PubMed] [Google Scholar]
- Daily JW, Reigel CE, Mishra PK, Jobe PC. The neurobiology of seizure predisposition in the genetically epilepsy-prone rats. Epilepsy Res. 1989;3:3–17. doi: 10.1016/0920-1211(89)90063-6. [DOI] [PubMed] [Google Scholar]
- Du W, Bautista JF, Yang H, Diez-Sampedro A, You SA, Wang L, et al. Calcium-sensitive channelopathy in human epilepsy and paroxysmal movement disorder. Nat Genet. 2005;37:733–738. doi: 10.1038/ng1585. [DOI] [PubMed] [Google Scholar]
- Ermolinsky B, Arshadmansab MF, Pacheco Otalora LF, Zarel MM, Garrido-Sanabria ER. Deficit of Kcnmal mRNA expression in the dentate gyrus of epileptic rats. NeuroReport. 2008;19:1291–1294. doi: 10.1097/WNR.0b013e3283094bb6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans MS, Cady CJ, Disney KE, Yang L, Laguardia JJ. Three brief epileptic seizures reduce inhibitory synaptic current, GABA (A) currents, and GABA (A)-receptor subunits. Epilepsia. 2006;47:1655–1664. doi: 10.1111/j.1528-1167.2006.00634.x. [DOI] [PubMed] [Google Scholar]
- Evans MS, Viola-McCabe KE, Caspary DM, Faingold CL. Loss of synaptic inhibition during repetitive stimulation in the genetically epilepsy-prone rats (GEPR) Epilepsy Res. 1994;18:97–105. doi: 10.1016/0920-1211(94)90002-7. [DOI] [PubMed] [Google Scholar]
- Evans MS, Cady CJ, Disney KE, Yang L, LaGuardia JJ. Three brief seizures reduce inhibitory synaptic currents, GABA(A) currents, and GABA(A) r-receptor subunits. Epilepsia. 2002;47:1655–1664. doi: 10.1111/j.1528-1167.2006.00634.x. [DOI] [PubMed] [Google Scholar]
- Faingold CL. Neuronal networks in the genetically epilepsy-prone rat. Adv Neurol. 1999;79:311–321. [PubMed] [Google Scholar]
- Faingold CL. Role of GABA abnormalities in the inferior colliculus pathophysiology - audiogenic seizures. Hear Res. 2002;168:223–227. doi: 10.1016/s0378-5955(02)00373-8. [DOI] [PubMed] [Google Scholar]
- Jobe PC, Picchioni AL, Chin L. Role of brain norepinephrine in audiogenic seizure in the rat. J Pharmacol Exp Ther. 1973;184:1–10. [PubMed] [Google Scholar]
- Köhler M, Hirschberg B, Bond CT, Kinzie JM, Marrion NV, Maylie J, Adelman JP. Small conductance, calcium-activated potassium channels from mammalian brain. Science. 1996;273:1709–1714. doi: 10.1126/science.273.5282.1709. [DOI] [PubMed] [Google Scholar]
- Lorenz S, Heils A, Kasper JM, Sander T. Allelic association of a truncation mutation of the KCNMB3 gene with idiopathic generalized epilepsy. Am J Med Genet Neuropsychiatr Genet. 2007;144B:10–13. doi: 10.1002/ajmg.b.30369. [DOI] [PubMed] [Google Scholar]
- Li Y, Evans MS, Faingold CL. In vitro electrophysiology of neurons in subnuclei of rat inferior colliculus. Hear Res. 1998;121:1–10. doi: 10.1016/s0378-5955(98)00066-5. [DOI] [PubMed] [Google Scholar]
- Molnar LR, Fleming WW, Taylor DA. Alterations in neuronal gamma-amino butyric acid(A) receptor responsiveness in genetic model of seizures susceptibility with different patterns. J Pharmacol Exp Ther. 2000;295:1258–1266. [PubMed] [Google Scholar]
- McCown TJ, Breese GR. Effects of apamin and nicotinic acetylcholine receptor antagonists on inferior collicular seizures. Eur J Pharmacol. 1990;187:49–58. doi: 10.1016/0014-2999(90)90339-8. [DOI] [PubMed] [Google Scholar]
- N’Gouemo P, Faingold CL. Periaqueductal gray neurons exhibit increased responsiveness associated with audiogenic seizures in the genetically epilepsy-prone rat. Neuroscience. 1998;84:619–25. doi: 10.1016/s0306-4522(97)00551-4. [DOI] [PubMed] [Google Scholar]
- N’Gouemo P, Faingold CL, Morad M. Calcium channel dysfunction in inferior colliculus neurons of the genetically epilepsy-prone rat. Neuropharmacology. 2009;56:665–675. doi: 10.1016/j.neuropharm.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- N’Gouemo P, Yasuda RP, Morad M, Faingold CL. Audiogenic seizure alters the expression of calcium and potassium channel protein in inferior colliculus neurons of the genetically epilepsy-prone rat (GEPR-3) Abs Soc Neurosci Program. 2003:212.20. [Google Scholar]
- N’Gouemo P, Yasuda RP, Morad M. Ethanol withdrawal is accompanied by downregulation of calcium channel alpha 1B subunit in rat inferior colliculus neurons. Brain Res. 2006;1108:216–220. doi: 10.1016/j.brainres.2006.06.028. [DOI] [PubMed] [Google Scholar]
- Pacheco Otalora LF, Hernandez EF, Arshadmansab MF, Francisco S, Willis M, Ermolinsky B, Zarei M, Knaus H-G, Garrido-Sanabria ER. Down-regulation of BK channel expression in the pilocarpine model of temporal lobe epilepsy. Brain Res. 2008;1200:116–131. doi: 10.1016/j.brainres.2008.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedarzani P, Mosbacher J, Rivard A, Cingolani LA, Oliver D, Stocker M, Adelman JP, Fakler B. Control of electrical activity in central neurons by modulating the gating of small conductance Ca2+-activated K+ channels. J Biol Chem. 2001;276:9762–9769. doi: 10.1074/jbc.M010001200. [DOI] [PubMed] [Google Scholar]
- Reid CA, Berkovic SF, Petrou S. Mechanisms of human inherited epilepsies. Prog Neurobiol. 2009 doi: 10.1016/j.pneurobio.2008.09.016. In press. [DOI] [PubMed] [Google Scholar]
- Sah P. Ca2+-activated K+ currents in neurones: types, physiological roles and modulation. Trends Neurosci. 1996;19:150–154. doi: 10.1016/s0166-2236(96)80026-9. [DOI] [PubMed] [Google Scholar]
- Savage DD, Reigel CE, Jobe PC. Angular bundle kindling is accelerated in rtas with a genetic predisposition to acoustic stimulus-induced seizures. Brain Res. 1986;376:412–415. doi: 10.1016/0006-8993(86)90211-8. [DOI] [PubMed] [Google Scholar]
- Sheehan JJ, Benedetti BL, Barth AL. Anticonvulsant effect of the BK-channels antagonist paxilline. Epilpesia **(*) 2008:1–10. doi: 10.1111/j.1528-1167.2008.01888.x. [DOI] [PubMed] [Google Scholar]
- Shruti S, Clem RL, Barth AL. A seizure-induced gain-of-function in BK channels is associated with elevated firing activity I neocortical pyramidal neurons. Neurobiol Dis. 2008;30:323–330. doi: 10.1016/j.nbd.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivaramakrishnan S, Oliver DL. Distinct potassium currents result in physiologically distinct cell types in inferior colliculus of the rat. J Neurosci. 2001;21:2861–2877. doi: 10.1523/JNEUROSCI.21-08-02861.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker M, Pedarzani P. Differential distribution of three Ca2+-activated K+ channel subunits, SK1, SK2, and SK3 in the adult rat central nervous system. Mol Cell Neurosci. 2000;15:476–493. doi: 10.1006/mcne.2000.0842. [DOI] [PubMed] [Google Scholar]
- Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of protein form polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma-Ahuja S, Pencek TL. Hippocampal CA1 neuronal properties in genetically epilepsy-prone rats: evidence of increased excitation. Epilepsy Res. 1994;18:205–215. doi: 10.1016/0920-1211(94)90041-8. [DOI] [PubMed] [Google Scholar]
- Verma-Ahuja S, Evans MS, Pencek TL. Evidence for decreased calcium dependent conductance in hippocampal CA3 neurons of genetically epilepsy-prone rats. Epilepsy Res. 1995;22:137–144. doi: 10.1016/0920-1211(95)00040-2. [DOI] [PubMed] [Google Scholar]
- Verma-Ahuja S, Evans MS, Espinosa JA. Evidence of increased excitability in GEPR hippocampus preceding development of seizure susceptibility. Epilepsy Res. 1998;31:161–173. doi: 10.1016/s0920-1211(98)00027-8. [DOI] [PubMed] [Google Scholar]
- Wang YH, Bosy TZ, Yasuda RP, Grayson DR, Vicini S, Pizzorusso T, Wolfe BB. Characterization of NMDA receptor subunit-specific antibodies: distribution of NR2A and NR2B receptor subunits in rat brain and ontogenic profile in the cerebellum. J Neurochem. 1995;65:176–183. doi: 10.1046/j.1471-4159.1995.65010176.x. [DOI] [PubMed] [Google Scholar]
- Williamson A, Spencer DD, Shepherd GM. Comparison between the membrane and synaptic properties of human and rodent dentate granule cells. Brain Res. 1993;622:194–202. doi: 10.1016/0006-8993(93)90819-9. [DOI] [PubMed] [Google Scholar]
- Xia XM, Fakler B, Rivard A, Wayman G, Johnson-Pais T, Keen JE, Ishii T, Hirschberg B, Bond CT, Lutsenko S, Maylie J, Adelman JP. Mechanism of calcium gating in small-conductance calcium-activated potassium channels. Nature. 1998;395:503–507. doi: 10.1038/26758. [DOI] [PubMed] [Google Scholar]