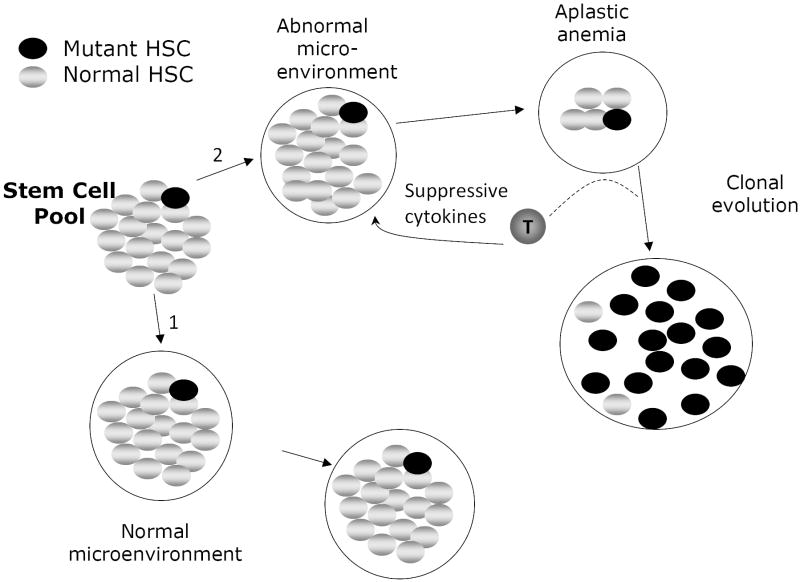
Figure 2.
Clonal evolution in acquired aplastic anemia. In the ground state, somatically mutated stem cells do not expand because the mutation doesn’t confer upon that cell a selective advantage. The coefficient of selection for clonal expansion of that cell is low. If the microenvironment remains normal over time (pathway 1), no clonal expansion occurs because the microenvironment is highly supportive of the majority of stem cells in the pool. That is, no clonal evolution occurs in pathway 1 because the relative fitness differences between the normal and mutant cells are trivial. If, however, T-lymphocytes arise that inhibit the replication, expansion or survival of normal hematopoietic stem cells, and if the somatic mutation confers upon a stem cell the capacity to resist the effect of the T cells (by resisting, for example, the apoptotic effects of suppressive cytokines like TNFα and IFNγ), then pathway 2 is most relevant. During the development of aplasia, normal stem cells are suppressed but the mutant one is not and, because the coefficient of selection for that cell is now high (as a result of a decline in fitness of the normal stem cells) it can expand clonally. In some cases the very cytokines that suppress the normal stem cells function to enhance the expansion of the mutant clone. Under continued pressure from the aberrant T cell population, the neoplastic clone preferentially expands over time while the less fit normal HSC are selected against. Not shown in this figure is a theoretical process by which a mutant stem cell arises in a population only after it is exposed to a hostile environment. This would meet strict genetic standards for a truly “adaptive mutation” in which the hostile environment per se induces mutations some of which permit an adaptive response to the environment. In light of the capacity of TNFα to induce oxidative DNA damage,82 this process is not simply a remote possibility. The coefficient of selection idea would still be relevant here as well because relief of the environmental stress (e.g. fully effective immunotherapy of aplastic anemia) might lower the coefficient in time to prevent an outgrowth of adapted clonal progeny. Finally, in some cases the new clone is able to subordinate the signals from the suppressive population and convert them to growth and survival signals (represented by the dashed line leading from the T-cell in pathway 2). For example, we have noted that in some cases of MDS arising in the context of bone marrow failure, TNFα enhances the proliferation of clonal erythroid and myeloid progenitors, a distinctly aberrant response.