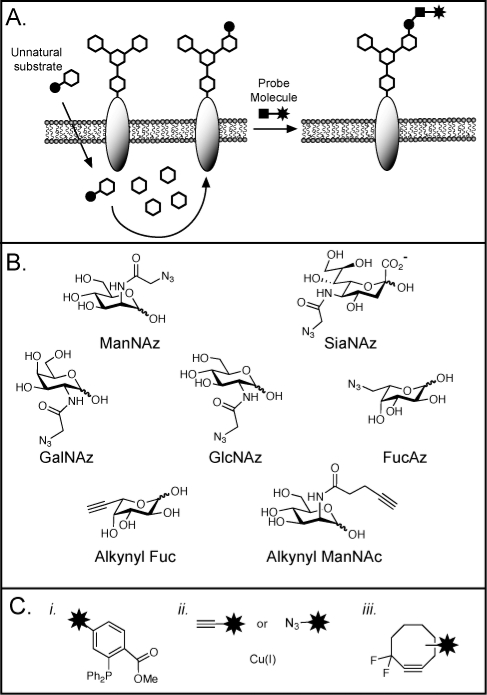
Figure 5.
The bioorthogonal chemical reporter strategy for profiling and visualizing glycans. (A) Metabolic labeling of cell-surface glycans with an azidosugar. The azide and alkyne functionalities can be covalently reacted with a probe that bears the functionality shown in panel C. (B) Azidosugars and alkynyl sugars that have been employed as metabolic labels. The unnatural monosaccharides are typically used in peracetylated form to facilitate cellular uptake. (C) Reagents for covalent labeling of azides (i, iii) and alkynes (ii): (i) phosphines for the Staudinger ligation; (ii) terminal alkynes or azides and copper(I) for copper-mediated azide−alkyne cylcoaddition, “click chemistry”; (iii) difluorinated cyclooctyne (DIFO) for strain-promoted cycloadditions.