Abstract
Background
The effects of oophorectomy on brain aging remain uncertain.
Methods
We conducted a cohort study with long-term follow-up of women in Olmsted County, Minn., USA, who underwent either unilateral or bilateral oophorectomy before the onset of menopause from 1950 through 1987. Each member of the oophorectomy cohort was matched by age to a referent woman from the same population who had not undergone any oophorectomy. We studied underlying and contributory causes of death in 1,274 women with unilateral oophorectomy, 1,091 women with bilateral oophorectomy, and 2,383 referent women.
Results
Mortality for neurological or mental diseases was increased in women who underwent bilateral oophorectomy before age 45 years compared with referent women (hazard ratio = 5.24; 95% confidence interval = 2.02–13.6; p < 0.001). Within this age stratum, mortality was similar in women who were or were not treated with estrogen from the time of oophorectomy through age 45 years, and in women who had bilateral oophorectomy for prophylaxis or for treatment of a benign ovarian condition. Mortality was also increased in women who underwent unilateral oophorectomy before age 45 years without concurrent hysterectomy.
Conclusions
Bilateral oophorectomy performed before age 45 years is associated with increased mortality for neurological or mental diseases.
Key Words: Oophorectomy, Neurological diseases, Mental diseases, Mortality, Cohort study
Introduction
The long-term effects of oophorectomy and of subsequent estrogen treatment on the brain remain uncertain and controversial [1,2,3,4,5]. In a previous article that focused on overall mortality [6], we reported increased mortality for neurological or mental disorders restricted to a cohort of 537 women who underwent bilateral oophorectomy for prophylaxis of cancer. However, the previous report did not consider the additional 554 women included in our study who underwent bilateral oophorectomy for a benign ovarian condition. In addition, we did not report specific causes of death for the 1,274 women included in our study who underwent unilateral oophorectomy, and we only considered one underlying cause of death for each woman [6]. However, more extensive information can be obtained by considering all causes of death listed anywhere on the death certificate [7].
To provide a more complete description of the full cohorts, to capture all diseases listed on the death certificate, and to group causes of death in specific neurological or mental disease categories, we conducted analyses including all neurological and mental diseases reported anywhere on the death certificates for all of the 4,748 women included in the Mayo Clinic Cohort Study of Oophorectomy and Aging [1,2,3, 6, 8]. Thus, we tested the hypothesis that the harmful effects of bilateral oophorectomy on the aging brain may be general and may involve several neuronal populations and brain structures. This hypothesis was recently detailed elsewhere [9].
Methods
Unilateral and Bilateral Oophorectomy Cohorts
The Mayo Clinic Cohort Study of Oophorectomy and Aging included a total of 1,274 Olmsted County, Minn., USA, women who underwent unilateral oophorectomy and 1,091 women who underwent bilateral oophorectomy during the 38-year period from 1950 through 1987 [6]. Oophorectomy was defined as complete removal of the ovary; further details about the two oophorectomy cohorts have been reported elsewhere [10,11,12,13]. All information about oophorectomy, including the indication defined by the gynecologist at the time of surgery, was abstracted from medical records included in the records linkage system of the Rochester Epidemiology Project [14]. Women were included in the current study if they were born before 1962 (at least age 40 years by January 1, 2002). In addition, we included only women who had undergone oophorectomy prior to menopause; however, we also included 158 women with unknown age at time of menopause who underwent bilateral oophorectomy before age 56 years (approximate upper limit of age at time of natural menopause). Finally, we excluded women who underwent either unilateral or bilateral oophorectomy as treatment for ovarian cancer, and women who underwent bilateral oophorectomy as treatment for another estrogen-related cancer [6].
Referent Cohort
The records linkage system of the Rochester Epidemiology Project provided the list of residents from which potential referent women were drawn. This list has been shown to be complete when compared with a random-digit-dialing telephone sample and with the census [14]. For each woman in the unilateral or in the bilateral oophorectomy cohorts, we defined the year of surgery as the index year. We then used simple random sampling to select 1 woman from the complete Olmsted County population with the same year of birth, who had survived without any oophorectomy to the index year. However, hysterectomy without oophorectomy was not an exclusion criterion, and referent women were not required to be premenopausal in the index year. All women in the population who met these criteria were considered eligible regardless of any possible diseases or risk factors (population-based referent sample).
Follow-up Procedures
The primary objective of follow-up in the Mayo Clinic Cohort Study of Oophorectomy and Aging was to detect incident cases of parkinsonism and dementia. Methods for tracking subjects and for detecting parkinsonism or dementia are reported elsewhere [1,2,3, 6, 8]. We describe here only the methods relevant to the assessment of causes of death. All study procedures were approved by the institutional review boards of the Mayo Clinic and Olmsted Medical Center. Both the oophorectomy and the referent cohorts were followed using three methods.
First, women were interviewed via telephone using a standardized questionnaire to assess their vital status and the presence of the diseases of interest. The telephone contact was direct whenever possible and conducted by 1 of 6 specifically trained research assistants. For deceased or incapacitated women (e.g., deaf, cognitively impaired, or terminally ill), we contacted a surrogate informant (proxy interview). Second, independent of the telephone contact, all women were followed passively through review of inpatient and outpatient medical records in the records linkage system of the Rochester Epidemiology Project [14]. Finally, vital status was assessed using state-specific or national death indices. For each deceased woman, we obtained a copy of the death certificate either from the records linkage system or from the vital statistics offices of individual states. When this was not possible, we obtained death certificate information from the National Death Index (NDI Plus; National Center for Health Statistics, Hyattsville, Md., USA).
A trained medical indexing clerk recoded all causes of death listed on the death certificates (underlying, intermediate, immediate, and other significant conditions) using the International Classification of Diseases, Eighth Revision (ICD-8), Adapted Codes for Hospitals (this is a modification of ICD-8 codes) [15]. The clerk was kept unaware of the oophorectomy status of deceased women to prevent bias. Causes of death were grouped into the following 7 categories: dementia, parkinsonism, multiple sclerosis (MS), amyotrophic lateral sclerosis (ALS), other neurologic diseases, psychosis, and other psychiatric diseases. The grouping was conducted by a neurologist (W.A.R.) who was kept unaware of the oophorectomy status of the women.
Statistical Analysis
We obtained survival curves using the Kaplan-Meier method and estimated hazard ratios (HRs) using Cox proportional hazards models. These models allowed for complete age-adjustment by using age as the time scale. The assumption of proportional hazards was assessed by graphical methods and by introducing a time-dependent coefficient in the Cox models [16].
We first considered deaths with neurological or mental diseases listed as the underlying cause. Second, we considered all deaths with neurological or mental diseases listed anywhere on the death certificate (including underlying, intermediate, and immediate causes of death, and other significant conditions contributing to death) [7]. We also considered mortality for specific neurological or mental diseases separately. However, because of the limited number of outcomes, these analyses were only exploratory.
Analyses were conducted overall and in strata defined by age at time of oophorectomy (or at index year) and by surgical indication. To simplify the presentation of results, we used arbitrary prespecified cut-offs for age (i.e., <45, 45–50, and >50 years) rather than distribution-related cut-offs (e.g., tertiles). However, the cut-off at age 50 years is also clinically relevant because it is the approximate median age of natural menopause [17]. We also conducted analyses accounting for the combined effect of age at time of bilateral oophorectomy and length of estrogen treatment after surgery. Information about treatment with oral or transdermal estrogen in women who underwent bilateral oophorectomy was abstracted from the inpatient and outpatient medical records in the records linkage system [14].
For women who underwent bilateral oophorectomy before age 45 years, we conducted a set of sensitivity analyses. To test the hypothesis that irreversible neurological damage may occur only many years after bilateral oophorectomy, we conducted sensitivity analyses excluding all women followed for less than 10 years. Additional sensitivity analyses excluded women who reported a history of either depression or anxiety before the index year or the 61 women with unknown age at the time of menopause. All analyses were completed using SAS® version 8.2 (SAS Institute, Cary, N.C., USA), and tests of statistical significance were conducted at the two-tailed alpha level of 0.05.
Results
Study Sample
Extensive details about the unilateral and bilateral oophorectomy cohorts and the referent cohort were reported elsewhere [6]. In brief, the 1,274 women in the unilateral oophorectomy cohort were followed for a median of 29.5 years (range = 54 days to 54.6 years); a total of 304 women died during follow-up, and 297 death certificates (98%) were obtained. The 297 death certificates yielded 1,106 distinct causes of death, and each cause received a diagnostic code (median = 3 causes of death per woman; range = 1–14). Three women died within 1 year of the oophorectomy. Of the 1,274 women included in these analyses, 628 women (49%) were interviewed directly, 190 women (15%) were interviewed via proxy, and the remaining 456 women (36%) had only passive follow-up information.
The 1,091 women included in the bilateral oophorectomy cohort were followed for a median of 25.0 years (range = 4 days to 53.8 years); a total of 360 died during follow-up, and 358 death certificates were obtained (99%). The 358 death certificates yielded 1,275 distinct causes of death (median = 3 causes of death per woman; range = 1–10). Nine women died within 1 year of the oophorectomy. Of the 1,091 women included in these analyses, 452 (41%) were interviewed directly, 232 (21%) were interviewed via proxy, and the remaining 407 women (37%) had only passive follow-up information.
Similarly, the 2,383 referent women included in the study were followed for a median of 26.4 years (range = 66 days to 55.1 years); 628 of these women died during follow-up, and 618 death certificates were obtained (98%). The 618 death certificates yielded 2,284 distinct causes of death (median = 3 causes of death per woman; range = 1–14). Of the 2,383 women included, 1,037 (44%) were interviewed directly, 443 (19%) via proxy, and 903 women (38%) had only passive follow-up information.
Overall Mortality for Neurological or Mental Diseases
Our results for death from all causes were reported elsewhere. In brief, we found an increased mortality from all causes and from noncancer causes in women who underwent bilateral oophorectomy before age 45 years [6].
Table 1 shows survival analyses restricted to neurological or mental diseases for women who underwent unilateral oophorectomy. Women who underwent unilateral oophorectomy did not experience increased mortality for neurological or mental diseases in overall analyses. The results were similar using only one underlying cause of death for each woman or using causes of death listed anywhere on the death certificate. By contrast, women who underwent unilateral oophorectomy before age 45 years without concurrent hysterectomy had an increased mortality.
Table 1.
Mortality associated with neurological and mental diseases following unilateral oophorectomy
| Cohort or stratum | Women at risk | Person- years of follow-up | Total deaths | Underlying cause of death1 |
Causes listed anywhere on the death certificate2 |
||||
|---|---|---|---|---|---|---|---|---|---|
| deaths | HR (95% CI) | p value | deaths | HR (95% CI) | p value | ||||
| Overall analyses | |||||||||
| Referent women | 2,383 | 62,285 | 628 | 50 (2.1%) | 1.00 (reference) | − | 115 (4.8%) | 1.00 (reference) | − |
| Women with unilateral oophorectomy | 1,274 | 37,499 | 304 | 24 (1.9%) | 0.90 (0.55–1.46) | 0.66 | 62 (4.9%) | 0.98 (0.72–1.33) | 0.88 |
| Benign conditions | 942 | 28,368 | 218 | 20 (2.1%) | 0.99 (0.59–1.66) | 0.96 | 51 (5.4%) | 1.06 (0.76–1.47) | 0.75 |
| No specified indication | 332 | 9,131 | 86 | 4 (1.2%) | 0.62 (0.22–1.71) | 0.35 | 11 (3.3%) | 0.73 (0.39–1.36) | 0.32 |
| With hysterectomy | 869 | 25,023 | 230 | 18 (2.1%) | 0.81 (0.47–1.39) | 0.45 | 46 (5.3%) | 0.88 (0.62–1.24) | 0.47 |
| Without hysterectomy | 405 | 12,476 | 74 | 6 (1.5%) | 1.31 (0.56–3.08) | 0.53 | 16 (4.0%) | 1.41 (0.83–2.40) | 0.20 |
| Analyses stratified by age at time of unilateral oophorectomy or index year | |||||||||
| Younger (<45 years) | |||||||||
| Referent women | 1,417 | 38,106 | 229 | 8 (0.6%) | 1.00 (reference) | − | 31 (2.2%) | 1.00 (reference) | − |
| Women with unilateral oophorectomy | 991 | 29,225 | 159 | 15 (1.5%) | 2.20 (0.93–5.20) | 0.07 | 35 (3.5%) | 1.34 (0.82–2.18) | 0.24 |
| Benign conditions | 773 | 23,277 | 127 | 12 (1.6%) | 2.07 (0.84–5.09)3 | 0.11 | 29 (3.8%) | 1.32 (0.79–2.19)3 | 0.29 |
| No specified indication | 218 | 5,948 | 32 | 3 (1.4%) | 3.50 (0.88–13.9)3 | 0.08 | 6 (2.8%) | 1.52 (0.63–3.68)3 | 0.35 |
| With hysterectomy | 628 | 17,990 | 109 | 10 (1.6%) | 1.89 (0.74–4.83)4 | 0.18 | 23 (3.7%) | 1.17 (0.68–2.03)4 | 0.56 |
| Without hysterectomy | 363 | 11,235 | 50 | 5 (1.4%) | 3.18 (1.03–9.81)4 | 0.04 | 12 (3.3%) | 1.79 (0.92–3.50)4 | 0.09 |
| Middle or older (≥45 years)5 | |||||||||
| Referent women | 966 | 24,179 | 399 | 42 (4.4%) | 1.00 (reference) | − | 84 (8.7%) | 1.00 (reference) | − |
| Women with unilateral oophorectomy | 283 | 8,275 | 145 | 9 (3.2%) | 0.51 (0.25–1.05) | 0.07 | 27 (9.5%) | 0.72 (0.47–1.12) | 0.15 |
Neurological or mental disease listed as the underlying cause of death on the death certificate.
Neurological or mental disease listed anywhere on the death certificate and including underlying, intermediate, immediate, or other significant conditions contributing to death.
A formal test of interaction was not significant for underlying cause of death (p = 0.55) and for causes of death listed anywhere on the death certificate (p = 0.79).
A formal test of interaction was not significant for underlying cause of death (p = 0.36) and for causes of death listed anywhere on the death certificate (p = 0.25).
The age classes 45–50 and >50 years were pooled in this table because few women underwent unilateral oophorectomy in these strata.
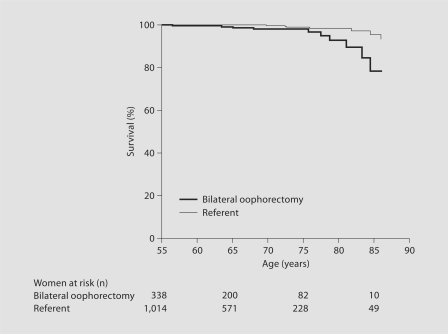
Table 2 shows survival analyses restricted to neurological or mental diseases for women who underwent bilateral oophorectomy. Analyses using only one underlying cause of death for each woman revealed a strong association in women who underwent bilateral oophorectomy before age 45 years (fig. 1). The association was similar in women who underwent oophorectomy before age 45 years but were treated with estrogen from the time of surgery through age 45 years or longer and in women who were not treated after surgery or interrupted their treatment before age 45 years. The association was also similar in women who underwent bilateral oophorectomy for prophylaxis of ovarian cancer and in those who had a benign ovarian condition prompting the oophorectomy. By contrast, we found no association for women who underwent oophorectomy between ages 45 and 50 years, or after age 50 years. A formal test of interaction between age at time of oophorectomy (or index year; continuous variable) and risk of death was not significant (p = 0.23 for underlying cause of death). When we considered all conditions listed anywhere on the death certificate, the association with neurological or mental diseases in women who underwent oophorectomy before age 45 years was attenuated and was not statistically significant (table 2).
Table 2.
Mortality associated with neurological and mental diseases following bilateral oophorectomy
| Cohort or stratum | Women at risk | Person- years of follow-up | Total deaths | Underlying cause of death1 |
Causes listed anywhere on the death certificate2 |
||||
|---|---|---|---|---|---|---|---|---|---|
| deaths | HR (95% CI) | p value | deaths | HR (95% CI) | p value | ||||
| Overall analyses | |||||||||
| Referent women | 2,383 | 62,285 | 628 | 50 (2.1%) | 1.00 (reference) | − | 115 (4.8%) | 1.00 (reference) | − |
| Women with bilateral oophorectomy | 1,091 | 27,864 | 360 | 34 (3.1%) | 1.20 (0.77–1.85) | 0.42 | 66 (6.1%) | 1.03 (0.76–1.40) | 0.84 |
| Prophylactic | 537 | 12,960 | 184 | 17 (3.2%) | 1.11 (0.64–1.92) | 0.72 | 34 (6.3%) | 0.99 (0.67–1.45) | 0.95 |
| Benign conditions | 554 | 14,904 | 176 | 17 (3.1%) | 1.30 (0.75–2.26) | 0.35 | 32 (5.8%) | 1.08 (0.73–1.60) | 0.71 |
| Analyses stratified by age at time of bilateral oophorectomy or index year3 | |||||||||
| Younger (<45 years) | |||||||||
| Referent women | 1,417 | 38,106 | 229 | 8 (0.6%) | 1.00 (reference) | − | 31 (2.2%) | 1.00 (reference) | − |
| Women with bilateral oophorectomy | 413 | 11,179 | 94 | 11 (2.7%) | 5.24 (2.02–13.6)4 | <0.001 | 16 (3.9%) | 1.80 (0.98–3.32)4 | 0.06 |
| Estrogen given from surgery until age 45 years or longer | 151 | 4,167 | 20 | 4 (2.7%) | 6.81 (1.91–24.3)5 | 0.003 | 5 (3.3%) | 1.97 (0.75–5.19)5 | 0.17 |
| Estrogen not given or discontinued before age 45 years | 262 | 7,012 | 74 | 7 (2.7%) | 4.67 (1.64–13.3)5 | 0.004 | 11 (4.2%) | 1.74 (0.87–3.47)5 | 0.12 |
| Prophylactic | 124 | 3,164 | 33 | 5 (4.0%) | 7.83 (2.48–24.8)6 | <0.001 | 7 (5.7%) | 2.69 (1.18–6.13)6 | 0.02 |
| Benign conditions | 289 | 8,015 | 61 | 6 (2.1%) | 4.39 (1.46–13.2)6 | 0.009 | 9 (3.1%) | 1.43 (0.68–3.02)6 | 0.35 |
| Middle (45–50 years) | |||||||||
| Referent women | 645 | 16,683 | 240 | 26 (4.0%) | 1.00 (reference) | − | 51 (7.9%) | 1.00 (reference) | − |
| Women with bilateral oophorectomy | 430 | 10,745 | 148 | 12 (2.8%) | 0.73 (0.37–1.46) | 0.37 | 24 (5.6%) | 0.75 (0.46–1.22) | 0.25 |
| Estrogen given from surgery until age 50 years or longer | 160 | 3,777 | 35 | 4 (2.5%) | 0.99 (0.34–2.84) | 0.98 | 8 (5.0%) | 0.99 (0.47–2.09) | 0.98 |
| Estrogen not given or discontinued before age 50 years | 270 | 6,968 | 113 | 8 (3.0%) | 0.65 (0.29–1.44) | 0.28 | 16 (5.9%) | 0.67 (0.38–1.18) | 0.16 |
| Older (>50 years) | |||||||||
| Referent women | 321 | 7,496 | 159 | 16 (5.0%) | 1.00 (reference) | − | 33 (10.3%) | 1.00 (reference) | − |
| Women with bilateral oophorectomy | 248 | 5,940 | 118 | 11 (4.4%) | 0.98 (0.45–2.12) | 0.95 | 26 (10.5%) | 1.09 (0.65–1.82) | 0.75 |
Neurological or mental disease listed as the underlying cause of death on the death certificate.
Neurological or mental disease listed anywhere on the death certificate and including underlying, intermediate, immediate, or other significant conditions contributing to death.
Formal tests of interaction between age at time of oophorectomy (or index year; continuous variable) were not statistically significant (p = 0.23 for underlying cause of death; p = 0.65 for causes of death listed anywhere on the death certificate).
For the sensitivity analysis restricted to women with 10 or more years of follow-up, results for underlying cause of death were the same (HR = 5.24; 95% CI = 2.02–13.6; p <0.001). This is because all 19 of the deaths (8 in referent women and 11 in women who underwent oophorectomy) were more than 10 years after the time of surgery or index. Results were also similar in the sensitivity analysis considering diagnoses listed anywhere on the death certificate (HR = 1.66; 95% CI = 0.89–3.09; p = 0.11). Sensitivity analyses excluding women who reported a history of either depression or of anxiety before the index year yielded an HR of 2.18 (95% CI = 0.69–6.88; p = 0.19) for underlying cause of death and 1.02 (95% CI = 0.43–2.41; p = 0.96) for causes of death listed anywhere on the death certificate (these analyses were restricted to 253 women with bilateral oophorectomy and 892 referent women who were interviewed). Sensitivity analyses excluding the 61 women with unknown age at time of menopause yielded an HR of 5.09 (95% CI = 1.93–13.40; p = 0.001) for underlying cause of death and 1.68 (95% CI = 0.89–3.17; p = 0.11) for causes of death listed anywhere on the death certificate.
A formal test of interaction was not significant for underlying cause of death (p = 0.56) and for causes of death listed anywhere on the death certificate (p = 0.82).
A formal test of interaction was not significant for underlying cause of death (p = 0.25) and for causes of death listed anywhere on the death certificate (p = 0.19).
Fig. 1.
Mortality for neurological or mental diseases in women who underwent bilateral oophorectomy before age 45 years compared with referent women. Only underlying causes of death were considered.
Sensitivity analyses restricted to women who had 10 or more years of follow-up yielded results similar to the corresponding primary analyses (table 2, footnote 4). Similarly, sensitivity analyses excluding women who reported a history of either depression or anxiety before the index year and analyses excluding the 61 women with unknown age at time of menopause yielded consistent results (table 2, footnote 4).
Specific Causes of Death
Table 3 shows exploratory survival analyses conducted separately for specific neurologic or mental diseases. Women who had bilateral oophorectomy experienced an increased mortality associated with ALS, MS, and parkinsonism. However, none of the specific comparisons reached statistical significance. We also compared the risk of suicide documented on the death certificate in women who underwent bilateral oophorectomy and in referent women. The hazard ratio (HR) was 2.14 (95% CI = 0.61–7.49; p = 0.23; total of 10 deaths) overall, and 2.18 (95% CI = 0.36–13.1; p = 0.40; total of 5 deaths) for women who underwent bilateral oophorectomy before age 45 years; however, none of the associations was statistically significant (data not shown in table).
Table 3.
Exploratory analyses for specific neurological or mental diseases following bilateral oophorectomy
| Cause of death | Underlying cause of death1 |
Causes listed anywhere on death certificate2 |
||||||
|---|---|---|---|---|---|---|---|---|
| referent cohort deaths | oophorectomy cohort deaths | HR (95% CI) | p value | referent cohort deaths | oophorectomy cohort deaths | HR (95% CI) | p value | |
| Dementia | 31 (1.3%) | 18 (1.7%) | 1.00 (0.56–1.79) | 0.99 | 68 (2.9%) | 37 (3.4%) | 0.94 (0.63–1.40) | 0.76 |
| Parkinsonism | 2 (0.1%) | 0 (0.0%) | Non-estimable | − | 7 (0.3%) | 9 (0.8%) | 2.32 (0.86–6.24) | 0.09 |
| Multiple sclerosis | 2 (0.1%) | 3 (0.3%) | 2.73 (0.46–16.4) | 0.27 | 2 (0.1%) | 3 (0.3%) | 2.73 (0.46–16.4) | 0.27 |
| ALS | 4 (0.2%) | 5 (0.5%) | 2.29 (0.61–8.52) | 0.22 | 4 (0.2%) | 5 (0.5%) | 2.29 (0.61–8.52) | 0.22 |
| Other neurological | 6 (0.3%) | 4 (0.4%) | 1.21 (0.34–4.31) | 0.76 | 18 (0.8%) | 13 (1.2%) | 1.40 (0.68–2.87) | 0.36 |
| Psychosis | 0 (0.0%) | 2 (0.2%) | Non-estimable | − | 6 (0.3%) | 3 (0.3%) | 0.96 (0.24–3.84) | 0.95 |
| Other psychiatric | 5 (0.2%) | 3 (0.3%) | 1.06 (0.25–4.44) | 0.94 | 20 (0.8%) | 8 (0.7%) | 0.73 (0.32–1.65) | 0.45 |
| Total | 50 (2.1%) | 34 (3.1%) | 1.20 (0.77–1.85) | 0.42 | 115 (4.8%) | 66 (6.1%) | 1.03 (0.76–1.40) | 0.84 |
Neurological or mental disease listed as the underlying cause of death on the death certificate.
Neurological or mental disease listed anywhere on the death certificate and including underlying, intermediate, immediate, or other significant conditions contributing to death.
Discussion
Our findings suggest that women who undergo bilateral oophorectomy at a young age are at increased risk of death associated with neurological or mental diseases. This increased mortality does not appear to be attenuated by estrogen treatment from the time of oophorectomy through age 45 years or longer. We are not aware of any other study investigating neurological or mental causes of death in women who underwent bilateral oophorectomy. Our first report from this same cohort study showed an increased risk of mortality for neurological or mental disorders in a cohort of 537 women who underwent bilateral oophorectomy for prophylaxis of cancer [6]. However, that report did not consider women who underwent bilateral oophorectomy prompted by a benign surgical indication and women who underwent unilateral oophorectomy. In addition, that study did not consider multiple causes of death for each woman [6].
Our findings for mortality are consistent with our findings for morbidity. We reported elsewhere that women who underwent bilateral oophorectomy at younger ages experienced an increased risk of parkinsonism, of cognitive impairment or dementia, and of depressive and anxiety symptoms [1,2,3, 8]. The findings reported here point to a possible additional association with ALS and MS. These diseases could not be studied as morbidity outcomes because they are rare.
Unfortunately, our findings for ALS and MS did not reach statistical significance. However, given the possible etiologic similarities between ALS, parkinsonism, and dementia, an association of oophorectomy with ALS is biologically plausible and deserves further investigation. The use of ALS mortality as a surrogate for ALS incidence is reasonable because the disease is severe and disabling, the duration is short, and the disease is generally recognized before death. ALS mortality derived from death certificates has been used as a surrogate for incidence by other investigators [18,19,20,21]. If confirmed by independent studies with a larger sample, these findings may implicate endocrinological mechanisms in the etiology of ALS in women. Similarly, estrogen treatment has been associated with less severe MS [22,23,24,25], and MS symptoms become less severe during pregnancy when estrogen levels are elevated [26]. Thus, it is biologically plausible that women with MS may have a worse prognosis when they undergo bilateral oophorectomy before menopause that results in a premature estrogen deficiency.
The finding of similar risks of death for neurological or mental diseases in women who underwent bilateral oophorectomy before age 45 years regardless of their subsequent treatment with estrogen from the time of oophorectomy through age 45 years suggests that mechanisms other than estrogen deficiency may be involved. Bilateral oophorectomy performed before menopause causes an abrupt deficiency of estrogen as well as of progesterone and testosterone. In addition, it causes a disruption of the hypothalamic-pituitary-ovarian axis which, in turn, causes an increase in gonadotropins (luteinizing hormone and follicle-stimulating hormone) [27]. It is possible that some of the harmful effects of bilateral oophorectomy on the brain are mediated by a deficiency in progesterone or testosterone or by an overproduction of gonadotropins [27,28,29,30,31,32,33]. This hypothesis deserves further investigation because it has major implications for the treatment of women who undergo early bilateral oophorectomy [9].
The finding of an increased mortality in women who underwent unilateral oophorectomy before age 45 years without concomitant hysterectomy remains of uncertain interpretation. It may be a chance finding or may indicate some hormonal consequences of unilateral oophorectomy (e.g., acceleration of ovarian failure in the remaining ovary) [2].
Strengths
There are several strengths to our study. First, our referent cohort was representative of the general population as demonstrated by a comparison with the life tables of Minnesota white women in corresponding census periods (628 observed deaths vs. 628.2 expected deaths; p = 0.99) [6]. This comparison also confirms that our methods to capture mortality were complete. Second, oophorectomy was documented historically in medical records, and no interview or recall of past surgical events was required [34]. Similarly, we were able to abstract information regarding estrogen treatment after bilateral oophorectomy from historical medical records, and no recall of past estrogen use was required [34]. Third, the women in the bilateral oophorectomy cohort were followed for a median of 25.0 years and those in the referent cohort were followed for a median of 26.4 years. Thus, we studied the long-term neurological and mental outcomes associated with bilateral oophorectomy. Fourth, the risk of misclassification of the underlying cause of death as coded on routine death certificates was reduced by considering all conditions listed anywhere on the death certificate for each woman [7].
Limitations
There are also limitations to our study. First, because of funding constraints, we could not study the incidence of all neurological or mental diseases using direct or proxy interviews or using medical record information from the records linkage system. The use of diseases mentioned anywhere on the death certificates was only a surrogate measure of the full manifestation of nonfatal and fatal disease. On the other hand, the consideration of all causes of death listed anywhere on the death certificate was a major improvement from the use of only one underlying cause of death for each woman [7].
Second, referent women were not required to be premenopausal in the index year. Thus, we may have biased our results toward the null (i.e., we may have observed HRs smaller than the true ones) if those women who experienced early natural menopause were at increased risk of death compared to women who reached natural menopause at later ages. However, the major findings of this study were restricted to women with index age of 45 years or younger, and in this age stratum the probability of an early natural menopause is low [35].
Third, we pooled into our primary analyses conditions that may have different etiologies and pathogeneses (e.g., psychosis and ALS). The rationale for these analyses is that bilateral oophorectomy and the resulting hormonal consequences may have a general detrimental effect on a wide range of metabolic and biochemical processes involved in the etiology of apparently disparate conditions. In support of a generalized effect of bilateral oophorectomy on the brain, we found associations with motor, cognitive, and psychiatric morbidity outcomes in this same study [1, 2, 8]. We recently reported an extensive discussion of the possible consequences of oophorectomy and its hormonal effects on several neuronal populations and brain structures [9].
Fourth, the sample size and the corresponding statistical power were inadequate to consider specific neurological or mental diseases separately. Similarly, the power was limited to study the modifying effect of estrogen treatment or to separate oral versus transdermal use of estrogen. In addition, there was some risk of false-positive findings due to multiple statistical testing.
Fifth, the association between bilateral oophorectomy and mortality for neurological or mental disorders may be confounded by socioeconomic status. In particular, lower socioeconomic status may increase the probability of undergoing a bilateral oophorectomy [36], may decrease the probability of receiving adequate estrogen treatment after the oophorectomy [37], and may independently increase mortality [38]. Unfortunately, information on income or education was not available for the overall cohorts. However, the population of Olmsted County is almost entirely middle-class, is well educated, and has excellent access to medical care [14]. In addition, the results were similar regardless of the indication for the oophorectomy. Because socioeconomic status may play a bigger role in a woman's decision to undergo bilateral oophorectomy for prophylaxis rather than as treatment for a benign ovarian condition, these stratified analyses are evidence against a major confounding effect (table 2).
Sixth, information about estrogen use among the referent women was not abstracted. Thus, our analyses on the effect of estrogen treatment were restricted to women with bilateral oophorectomy. Interestingly, only 60% of the women who underwent bilateral oophorectomy were ever prescribed estrogen treatment [11]. The majority of women were treated with unopposed estrogen (conjugated equine estrogen therapy in 82% of the women) [11].
Seventh, unfortunately some of the findings using underlying cause of death were not replicated using all causes of death listed anywhere on the death certificate. In general, the associations were attenuated when using multiple causes of death. The reasons for these discrepancies remain uncertain but may suggest an effect of oophorectomy on severity of disease manifestation. As expected, mental causes of death were more commonly considered contributory causes of death rather than underlying causes of death.
Conclusions
This study showed that women who underwent bilateral oophorectomy before age 45 years were at increased risk of neurological or mental diseases. This risk was not modified by estrogen treatment from the time of oophorectomy through age 45 years. Our preliminary findings suggest a possible association of bilateral oophorectomy with ALS or MS that deserves further investigation.
Acknowledgment
This work is supported by grants from the National Institute on Aging (R01 NS33978) and the National Institute of Arthritis and Musculoskeletal and Skin Diseases (R01 AR30582).
References
- 1.Rocca WA, Bower JH, Maraganore DM, Ahlskog JE, Grossardt BR, de Andrade M, Melton LJ., 3rd Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology. 2007;69:1074–1083. doi: 10.1212/01.wnl.0000276984.19542.e6. [DOI] [PubMed] [Google Scholar]
- 2.Rocca WA, Bower JH, Maraganore DM, Ahlskog JE, Grossardt BR, de Andrade M, Melton LJ., 3rd Increased risk of parkinsonism in women who underwent oophorectomy before menopause. Neurology. 2008;70:200–209. doi: 10.1212/01.wnl.0000280573.30975.6a. [DOI] [PubMed] [Google Scholar]
- 3.Rocca WA, Grossardt BR, Maraganore DM. The long-term effects of oophorectomy on cognitive and motor aging are age dependent. Neurodegener Dis. 2008;5:257–260. doi: 10.1159/000113718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shuster LT, Gostout BS, Grossardt BR, Rocca WA. Prophylactic oophorectomy in premenopausal women and long-term health. Menopause Int. 2008;14:111–116. doi: 10.1258/mi.2008.008016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shumaker SA, Legault C, Kuller L, Rapp SR, Thal L, Lane DS, Fillit H, Stefanick ML, Hendrix SL, Lewis CE, Masaki K, Coker LH. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women's Health Initiative Memory Study. JAMA. 2004;291:2947–2958. doi: 10.1001/jama.291.24.2947. [DOI] [PubMed] [Google Scholar]
- 6.Rocca WA, Grossardt BR, de Andrade M, Malkasian GD, Melton LJ., 3rd Survival patterns after oophorectomy in premenopausal women: a population-based cohort study. Lancet Oncol. 2006;7:821–828. doi: 10.1016/S1470-2045(06)70869-5. [DOI] [PubMed] [Google Scholar]
- 7.Porta MS, International Epidemiological Association . A Dictionary of Epidemiology. ed 5. Oxford: Oxford University Press; 2008. [Google Scholar]
- 8.Rocca WA, Grossardt BR, Geda YE, Gostout BS, Bower JH, Maraganore DM, de Andrade M, Melton LJ., 3rd Long-term risk of depressive and anxiety symptoms after early bilateral oophorectomy. Menopause. 2008;15:1050–1059. doi: 10.1097/gme.0b013e318174f155. [DOI] [PubMed] [Google Scholar]
- 9.Rocca WA, Shuster LT, Grossardt BR, Maraganore DM, Gostout BS, Geda YE, Melton LJ., 3rd Long-term effects of bilateral oophorectomy on brain aging: unanswered questions from the Mayo Clinic Cohort Study of Oophorectomy and Aging. Womens Health (London) 2009;5:39–48. doi: 10.2217/17455057.5.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Melton LJ, 3rd, Bergstralh EJ, Malkasian GD, O'Fallon WM. Bilateral oophorectomy trends in Olmsted County, Minnesota, 1950–1987. Epidemiology. 1991;2:149–152. [PubMed] [Google Scholar]
- 11.Melton LJ, 3rd, Crowson CS, Malkasian GD, O'Fallon WM. Fracture risk following bilateral oophorectomy. J Clin Epidemiol. 1996;49:1111–1115. doi: 10.1016/0895-4356(96)00211-9. [DOI] [PubMed] [Google Scholar]
- 12.Melton LJ, 3rd, Khosla S, Malkasian GD, Achenbach SJ, Oberg AL, Riggs BL. Fracture risk after bilateral oophorectomy in elderly women. J Bone Miner Res. 2003;18:900–905. doi: 10.1359/jbmr.2003.18.5.900. [DOI] [PubMed] [Google Scholar]
- 13.Beard CM, Crowson CS, Malkasian GD, O'Fallon WM, Melton LJ., 3rd Cardiovascular disease and cancer risk following bilateral oophorectomy: a population-based study in Rochester, Minnesota. J Womens Health. 1995;4:133–141. [Google Scholar]
- 14.Melton LJ., 3rd History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71:266–274. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 15.Commission on Professional and Hospital Activities: H-ICDA, Hospital Adaptation of ICDA, ed 2. Ann Arbor, 1973.
- 16.Therneau TM, Grambsch PM. Modeling Survival Data: Extending the Cox Model. New York: Springer; 2000. [Google Scholar]
- 17.Armstrong K, Schwartz JS, Randall T, Rubin SC, Weber B. Hormone replacement therapy and life expectancy after prophylactic oophorectomy in women with BRCA1/2 mutations: a decision analysis. J Clin Oncol. 2004;22:1045–1054. doi: 10.1200/JCO.2004.06.090. [DOI] [PubMed] [Google Scholar]
- 18.Weisskopf MG, O'Reilly EJ, McCullough ML, Calle EE, Thun MJ, Cudkowicz M, Ascherio A. Prospective study of military service and mortality from ALS. Neurology. 2005;64:32–37. doi: 10.1212/01.WNL.0000148649.17706.D9. [DOI] [PubMed] [Google Scholar]
- 19.Buckley J, Warlow C, Smith P, Hilton-Jones D, Irvine S, Tew JR. Motor neuron disease in England and Wales, 1959–1979. J Neurol Neurosurg Psychiatry. 1983;46:197–205. doi: 10.1136/jnnp.46.3.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chio A, Magnani C, Oddenino E, Tolardo G, Schiffer D. Accuracy of death certificate diagnosis of amyotrophic lateral sclerosis. J Epidemiol Community Health. 1992;46:517–518. doi: 10.1136/jech.46.5.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoffman PM, Brody JA. The reliability of death certificate reporting for amyotrophic lateral sclerosis. J Chronic Dis. 1971;24:5–8. doi: 10.1016/0021-9681(71)90053-1. [DOI] [PubMed] [Google Scholar]
- 22.Smith R, Studd JW. A pilot study of the effect upon multiple sclerosis of the menopause, hormone replacement therapy and the menstrual cycle. J R Soc Med. 1992;85:612–613. doi: 10.1177/014107689208501008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holmqvist P, Wallberg M, Hammar M, Landtblom AM, Brynhildsen J. Symptoms of multiple sclerosis in women in relation to sex steroid exposure. Maturitas. 2006;54:149–153. doi: 10.1016/j.maturitas.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 24.Voskuhl RR. Hormone-based therapies in MS. Int MS J. 2003;10:60–66. [PubMed] [Google Scholar]
- 25.Sicotte NL, Liva SM, Klutch R, Pfeiffer P, Bouvier S, Odesa S, Wu TC, Voskuhl RR. Treatment of multiple sclerosis with the pregnancy hormone estriol. Ann Neurol. 2002;52:421–428. doi: 10.1002/ana.10301. [DOI] [PubMed] [Google Scholar]
- 26.Houtchens MK. Pregnancy and multiple sclerosis. Semin Neurol. 2007;27:434–441. doi: 10.1055/s-2007-991127. [DOI] [PubMed] [Google Scholar]
- 27.Morrison JH, Brinton RD, Schmidt PJ, Gore AC. Estrogen, menopause, and the aging brain: how basic neuroscience can inform hormone therapy in women. J Neurosci. 2006;26:10332–10348. doi: 10.1523/JNEUROSCI.3369-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gibbs RG. Long-term treatment with estrogen and progesterone enhances acquisition of a spatial memory task by ovariectomized aged rats. Neurobiol Aging. 2000;21:107–116. doi: 10.1016/s0197-4580(00)00103-2. [DOI] [PubMed] [Google Scholar]
- 29.Webber KM, Casadesus G, Marlatt MW, Perry G, Hamlin CR, Atwood CS, Bowen RL, Smith MA. Estrogen bows to a new master: the role of gonadotropins in Alzheimer pathogenesis. Ann N Y Acad Sci. 2005;1052:201–209. doi: 10.1196/annals.1347.020. [DOI] [PubMed] [Google Scholar]
- 30.Singh M. Progesterone-induced neuroprotection. Endocrine. 2006;29:271–274. doi: 10.1385/ENDO:29:2:271. [DOI] [PubMed] [Google Scholar]
- 31.Singh M, Sumien N, Kyser C, Simpkins JW. Estrogens and progesterone as neuroprotectants: what animal models teach us. Front Biosci. 2008;13:1083–1089. doi: 10.2741/2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brinton RD, Thompson RF, Foy MR, Baudry M, Wang J, Finch CE, Morgan TE, Pike CJ, Mack WJ, Stanczyk FZ, Nilsen J. Progesterone receptors: form and function in brain. Front Neuroendocrinol. 2008;29:313–339. doi: 10.1016/j.yfrne.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bialek M, Zaremba P, Borowicz KK, Czuczwar SJ. Neuroprotective role of testosterone in the nervous system. Pol J Pharmacol. 2004;56:509–518. [PubMed] [Google Scholar]
- 34.Beard CM, Melton LJ, 3rd, Cedel SL, Richelson LS, Riggs BL. Ascertainment of risk factors for osteoporosis: comparison of interview data with medical record review. J Bone Miner Res. 1990;5:691–699. doi: 10.1002/jbmr.5650050705. [DOI] [PubMed] [Google Scholar]
- 35.Coulam CB, Adamson SC, Annegers JF. Incidence of premature ovarian failure. Obstet Gynecol. 1986;67:604–606. [PubMed] [Google Scholar]
- 36.Marks NF, Shinberg DS. Socioeconomic status differences in hormone therapy. Am J Epidemiol. 1998;148:581–593. doi: 10.1093/oxfordjournals.aje.a009684. [DOI] [PubMed] [Google Scholar]
- 37.Grodstein F, Clarkson TB, Manson JE. Understanding the divergent data on postmenopausal hormone therapy. N Engl J Med. 2003;348:645–650. doi: 10.1056/NEJMsb022365. [DOI] [PubMed] [Google Scholar]
- 38.Lynch JW, Kaplan GA, Cohen RD, Tuomilehto J, Salonen JT. Do cardiovascular risk factors explain the relation between socioeconomic status, risk of all-cause mortality, cardiovascular mortality, and acute myocardial infarction? Am J Epidemiol. 1996;144:934–942. doi: 10.1093/oxfordjournals.aje.a008863. [DOI] [PubMed] [Google Scholar]