Abstract
Craniometaphyseal dysplasia (CMD) is a monogenic human disorder characterized by thickening of craniofacial bones and flaring metaphyses of long bones. Mutations for autosomal dominant CMD have been identified in the progressive ankylosis gene ANKH. Previous studies of Ank loss-of-function models, Anknull/null and Ankank/ank mice, suggest that Ank plays a role in the regulation of bone mineralization. However, the mechanism for Ank mutations leading to CMD remains unknown. We generated the first knockin (KI) mouse model for CMD expressing a human mutation (Phe377 deletion) in ANK. Homozygous Ank knockin mice (AnkKI/KI) replicate many typical features of human CMD including hyperostosis of craniofacial bones, massive jawbones, decreased diameters of cranial foramina, obliteration of nasal sinuses, fusion of middle ear bones, and club-shaped femurs. In addition, AnkKI/KI mice have increased serum alkaline phosphatase and TRACP5b, as reported in CMD patients. Biochemical markers of bone formation and bone resorption, N-terminal propeptide of type I procollagen and type I collagen cross-linked C-terminal telopeptide, are significantly increased in AnkKI/KI mice, suggesting increased bone turnover. Interestingly, AnkKI/KI bone marrow–derived macrophage cultures show decreased osteoclastogenesis. Despite the hyperostotic phenotype, bone matrix in AnkKI/KI mice is hypomineralized and less mature, indicating that biomechanical properties of bones may be compromised by the Ank mutation. We believe this new mouse model will facilitate studies of skeletal abnormalities in CMD at cellular and molecular levels.
Key words: craniometaphyseal dysplasia, Ank, skeletal phenotype, genetic disorder, biochemical marker
INTRODUCTION
Craniometaphyseal dysplasia (CMD) is characterized by progressive thickening of craniofacial bones and flaring metaphyses with increased radiolucency of long bones. CMD can be diagnosed early in infants and progresses throughout life.(1,2) Hyperostosis of skulls frequently leads to obstruction of cranial nerve foramina. The common consequences of neuronal compression in CMD patients are hearing loss, visual impairment, and facial palsy. Treatment of severe cases of CMD is limited to surgical intervention to decompress obstructed foramina and to correct craniofacial structures. Treatment with calcitonin, which inhibits bone turnover, can correct biochemical abnormalities but has no effect on cranial hyperostosis.(3,4) Calcitriol, a stimulator of bone resorption, in combination with low calcium diet, can improve facial paralysis but has no effect on abnormal metaphyses.(5)
Knowledge of CMD pathoetiology is based on a few case reports, because there has been no animal model available. Previous studies showed normal or decreased serum calcium and phosphorus levels, normal to slightly elevated PTH, and increased serum alkaline phosphatase (ALP), and TRACP in CMD patients.(3,6–9) Histopathological studies of CMD patients showed either increased osteoblast numbers, no osteoclasts in periosteal or endosteal layers, or increased osteoblast and osteoclast numbers.(3,5,10–12) One paper showed decreased osteoclastogenesis in in vitro osteoclast-like cultures derived from bone marrow of a CMD patient.(13) Altogether, these findings are inconsistent and thus the precise pathophysiology of CMD remains obscure.
CMD occurs sporadically or is transmitted as an autosomal dominant (AD) or autosomal recessive (AR) trait. AD CMD is more common and less severe than the AR form.(14) Mutations for AD CMD have been identified in the human ANK gene (ANKH) in form of point mutations—one amino acid insertions or deletions that cluster mostly in cytoplasmic domains close to the C terminus.(15,16) Mutations in the N terminus of ANKH result in another human skeletal disorder, familial calcium pyrophosphate dihydrate deposition disease (CPPDD), characterized by calcification of cartilage and periarticular tissues caused by deposition of CPPD crystals.(17–19) Unlike in CMD, CPPDD patients have no apparent defect in skulls or long bones.
Ank is a 492 amino acid protein with 10 or 12 transmembrane domains and is present on plasma membranes, endoplasmic reticulum, Golgi, and mitochondria.(20,21) A highly conserved sequence within vertebrates and its wide expression in skeletal and nonskeletal tissues suggest an important function of Ank.(16,20) Two Ank loss-of-function models, Ank ank/ank and Ank null/null mice, share a remarkable similarity in joint and skeletal phenotypes.(22) Ank ank/ank mice carry a premature stop codon in the C terminus and show progressive arthritic destruction of joints with increased hydroxyapatite (HA) deposition, which eventually leads to joint fusions.(23,24) ANK serves as a PPi transporter to channel intracellular PPi to extracellular matrix, thus regulating mineralization in bones and preventing ectopic calcification.(20) Fibroblasts from Ank ank/ank mice show a decrease in extracellular pyrophosphate (ePPi) levels and an increase in intracellular PPi (iPPi) levels compared with wildtype cells.(20)
Although Ank null/null mice develop cranial hyperostosis and narrowing of foramen magnum, several human CMD characteristics such as hyperostotic mandibles, obstruction of nasal sinuses, and flaring metaphyses of femurs are not replicated in these mice.(25) To gain insights into the pathogenesis of craniometaphyseal dysplasia (CMD), we generated a knockin (KI) mouse model expressing one of the most common mutations identified in the AD form of CMD, an in-frame deletion of phenylalanine 377 (Phe377del) in ANK. Our skeletal and biochemical analyses show that Ank knockin mice display many CMD-like features and are therefore a useful model for CMD.
MATERIALS AND METHODS
Mice
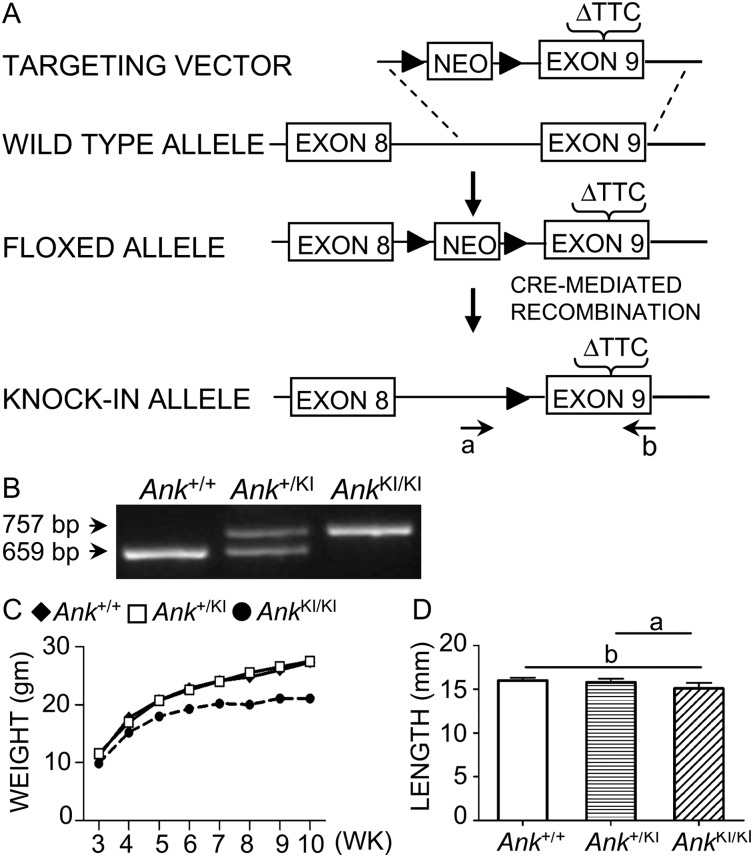
We generated a knockin mouse model in the Gene Targeting and Transgenic Facility (GTTF) at UCHC introducing a deletion of TTC1130–1132 (phenylalanine 377) into exon 9 of Ank. The TTC deletion was created in a fragment of BAC RP23–296N12 (CHORI, Oakland, CA, USA), which was retrieved in a PL253 vector according to established protocols (http://recombineering.ncifcrf.gov/) and consisted of a 7.1-kb 5′-arm and a 3.2-kb fragment extending 3′ of the mutation. A floxed PGK-Neo cassette was inserted 608 bp 5′ of the TTC deletion. The construct was introduced into 129/Sv embryonic stem cells through recombineering (Fig. 1A). Founder mice were crossed with HPRT1-Cre deleter mice to eliminate a floxed neomycin sequence used for selection of ES cells. Transmission of the mutant allele was confirmed by sequencing. PCR genotyping with tail DNA generates a 659-bp product for wildtype and a 757-bp product for mutant Ank (Fig. 1B). The forward primer (5′-GCTAAGCTTCCATACTTACCCGTCTGC-3′) is located 5′ of the remaining loxP site, and the reverse primer (5′-CCTGCCCCTTACCTGGCACTG-3′) is located 3′ of the TTC deletion. In knockin mice, the integrity of the intron preceding exon 9 is maintained except for the presence of a 101-bp fragment containing the remaining loxP site and a short fragment from a multiple cloning site. The animal protocol was approved by the Animal Care Committee of the University of Connecticut Health Center, and all work was performed in an AAALAC-accredited facility under veterinary supervision. Mice were bred from a 129/Sv into a C57Bl/J6 background (N5) for skeletal analysis. Ank ank/ank mice used for skeletal analyses were in C3F3B6A/Aw-J background (Jackson Laboratories).
FIG. 1.
Generation and genotyping of Ank KI/KI mice. (A) Deletion of phenylalanine 377 in exon 9 introduced into the mouse Ank gene by homologous recombination. The floxed allele contains a PGK-Neo cassette (loxP indicated by solid triangle) and a TTC1130–1132 deletion in exon 9. The knockin allele after cre-mediated recombination contains one loxP site upstream of mutant exon 9. Genotyping primers (a and b) flank the loxP and the deletion site. (B) PCR genotyping assay for Ank +/+, Ank +/KI, and Ank KI/KI animals. Wildtype allele: 659 bp, mutant allele: 757 bp. (C) Total body weight measurement of Ank +/+, Ank +/KI, and Ank KI/KI male mice at 3–10 wk of age (n ≥ 6). (D) Femur length of 10-wk-old Ank +/+ (n = 12), Ank +/KI (n = 11), and Ank KI/KI (n = 9) male mice; a p < 0.05 and b p < 0.01 indicate statistical significance by one-way ANOVA.
Skeletal analysis
Radiographs of skulls, mandibles, and femurs of Ank+/+, Ank+/KI, and AnkKI/KI male mice at 1, 3, and 6 mo of age (n > 3 for each group) were obtained by a MX20 Radiography System (Faxitron X-ray). BMC and BMD of skulls, mandibles, and femurs from 10-wk-old Ank+/+ (n ≥ 8) and AnkKI/KI (n ≥ 6) mice were determined by DXA using a Lunar PIXImus densitometer (Lunar). Skulls, mandibles, and femurs from 3-mo-old Ank+/+ (n = 5) and AnkKI/KI (n = 7) male mice were analyzed using μCT in the MicroCT facility at UCHC (mCT20; ScanCo Medical, Bassersdorf, Switzerland). We also examined Ankank/ank mice (n = 5) and their wildtype littermates (n = 5). Calvariae were analyzed over an area of 100 slices using the sagittal suture of the central parietal region as reference point. Mandibular data were collected by measuring vertical sections at the mandibular foramen. Trabecular measurements of femurs were taken at the distal growth plate in 80 consecutive slices of 12-μm resolution over a distance of 960 μm. Volumetric regions were rendered as 3D arrays with an isometric voxel dimension of 12 μm. Fifty cross-sectional slices of 12 μm in the mid-diaphysis were used to calculate cortical bone parameters.
Biochemical analysis
Sera were prepared from 10-wk-old fasted Ank +/+ (n ≥ 6) and Ank KI/KI (n ≥ 7) male mice. Serum TRACP5b (TRACP5b ELISA kit; IDS), type I collagen cross-linked C-terminal telopeptide (CTX; Ratlaps ELISA kit; Nordic Bioscience), and propeptide of type I procollagen (P1NP; rat/mouse P1NP kit; IDS) were measured according to the manufacturers' instructions. ALP activity was determined directly from serum by a colorimetric method using p-nitrophenol phosphate, which is hydrolyzed by ALP into p-nitrophenol.(26) Briefly, 15 μl of serum was added to substrate solution containing 15 mM 4-nitrophenyl phosphate in 1 M diethanolamine and 0.5 mM MgCl2 (pH 9.8). Absorbance was read at 405 nm after a 5-min incubation.
Bone histomorphometry
We injected Ank +/+ (n = 8) and Ank KI/KI (n = 10) male mice intraperitoneally with calcein (10 mg/kg body weight) and xylenol orange (90 mg/kg body weight) at an interval of 7 days. Two days after the second injection, mice were killed at 10 wk of age, and bones were subjected to histomorphometry as described.(27) For static histomorphometry, calvariae and femurs were fixed in 4% PFA and decalcified in 14% EDTA. Series of 5-μm paraffin sections were stained for TRACP. Osteoblast and osteoclast numbers in an area between 400 and 2,000 μm distal to the growth plate–metaphyseal junction of the distal femur were counted and normalized to the trabecular bone surface. For dynamic histomorphometry, frozen tissues in OCT (Richard-Allan Scientific) were sectioned with a cryotome (CM3050S; Leica). Measurements were obtained by BIOQUANT (BIOQUANT Image Analysis) and OsteoMeasure software (OsteoMetrics) for static and dynamic histomorphometry.
Ash weight
Femurs from 10-wk-old Ank +/+ (n = 11) and Ank KI/KI (n = 11) male mice had been defleshed by Dermestid beetles. Metaphyseal regions and trabeculae were removed from diaphyseal segments. Cortical bones were dried at 105–110°C for 15–17 h, dry weight was measured, and bones were ashed at 600°C for 18–20 h. Mineral content was calculated as the ratio of ash weight to dry weight.
Fourier-transform infrared spectroscopy
Femurs from 10-wk-old Ank +/+ (n = 4) and Ank KI/KI (n = 6) male mice were embedded in polymethyl methacrylate (PMMA). Three-micrometer longitudinal sections were mounted on barium fluoride (BaF2) infrared windows (Spectral Systems) and examined by Fourier-transform infrared (FTIR) microspectroscopy and imaging using a PerkinElmer Spotlight Imaging System (Perkin Elmer Instruments). A 400 × 400-μm area of cortical bone at the proximal metaphysis was scanned with 6.25 μm spatial resolution, 4 cm−1 spectral resolution, and 16 scans/pixel. All spectra were baseline-corrected, and the background spectrum contribution of PMMA was subtracted and processed by ISYS Chemical Imaging Software (v5.0; Spectral Dimensions). The mineral-to-matrix ratio was calculated by the ratio of the integrated area under the phosphate (916–1180 cm−1) and amide I bands (1585–1720 cm−1). Collagen cross-linking was calculated from the intensity ratio of sub-bands at 1660 and 1690 cm−1. Carbonate to phosphate ratio was calculated as area ratio of carbonate (840–900 cm−1) and phosphate bands (916–1180 cm−1). Mineral crystallinity was calculated from the intensity ratio of 1030/1020 cm−1. Data were collected from the center cortex of femurs.
X-ray diffraction
Tibia and scapulae were cleaned of adherent tissues and stored at −20°C before analysis. They were ground in a liquid nitrogen cooled mill (Spex Industries) and subjected to wide-angle X-ray diffraction on a Bruker AX8 diffractometer with Ni-filtered CuK α radiation. Scans were run from 4° 2 θ to 50° 2 theta. The particle size in the c-axis direction was estimated by line-broadening analysis of the 002 peak (25.85° 2 θ) using instrument-provided software. The full width at half-maximum is linearly related to the crystallite size and perfection (t) based on the Debye Scherrer equation.
In vitro osteoclast assays
We used mouse bone marrow–derived macrophage cultures (BMMs) to examine osteoclast formation and function. Briefly, bone marrow was flushed out from femora and tibia of 7- to 9-wk-old mice. Cells were cultured for 18–24 h in αMEM containing 10% FBS (Hyclone), 100 IU/ml penicillin, and 100 μg/ml streptomycin (Gibco Invitrogen). Nonadherent cells were collected and purified by Ficoll separation (Lymphoprep; Axis Shield, Oslo, Norway). Cells were seeded at a density of 5000 cells/well on 96-well culture plates in αMEM with 10% FBS, murine macrophage colony-stimulating factor (mM-CSF; 30 ng/ml; R&D Systems), and mRANKL (30 ng/ml; R&D System) to stimulate osteoclast differentiation. At indicated time points, cells were fixed with 2.5% glutaraldehyde and stained with TRACP (Lymphocyte Acid Phosphatase Kit; Sigma). The TRACP+ cells (nuclei ≥ 3) were counted as mature osteoclasts. For resorption assays, BMMs were cultured for 12 days on calcium phosphate–coated slides (Osteologic Discs; BD Biosciences) and bone chips. Osteologic discs were von Kossa stained and photographed under a dissecting microscope. Resorption pits appear white, contrasting to the black background of remaining mineral. To analyze the resorption pit on bone chips, 12 images for each bone chip were taken using a scanning electronic microscope (TM-1000 Tabletop Microscope; Hitachi High Technologies America). Percent resorption was calculated as the ratio of resorbed area to total area using Adobe Photoshop software for osteologic discs and Digimizer software for bone chips.
Statistical analysis
Statistical analysis was performed by Student's t-test or one-way ANOVA as indicated, using Prism 5 software (GraphPad Software).
RESULTS
General appearance of AnkKI/KI mice
Ank KI/KI mice appeared normal at birth but developed stiffness of joints at age of 4–5 wk. Similar to Ank ank/ank and Ank null/null mice, they died around 6 mo of age from an unknown cause. After weaning, Ank KI/KI mice weighed less and had shorter body length than their respective wildtype and heterozygous littermates (Fig. 1C, data not shown). A small but significant decrease in femur length of Ank KI/KI mice was observed at the age of 10 wk (Fig. 1D).
CMD-like skeletal phenotype of AnkKI/KI mice
Diagnosis of CMD in humans is based on clinical and radiographic findings.(11,28) We examined skeletal elements of 1-, 3-, and 6-mo-old Ank +/+, Ank +/KI, and Ank KI/KI male mice by X-ray imaging. As in CMD patients, Ank KI/KI mice showed increased radiopacity of skulls and mandibles (Fig. 2A). In addition, they exhibited club-shaped femurs with increased radiolucency in metaphyses at all ages examined. In Ank KI/KI mice, this CMD-like phenotype appeared early and progressed with age. Ank +/KI mice were phenotypically normal but developed an intermediate phenotype as they aged. This observation suggests that the effect of the Ank mutation may be dose and time dependent.
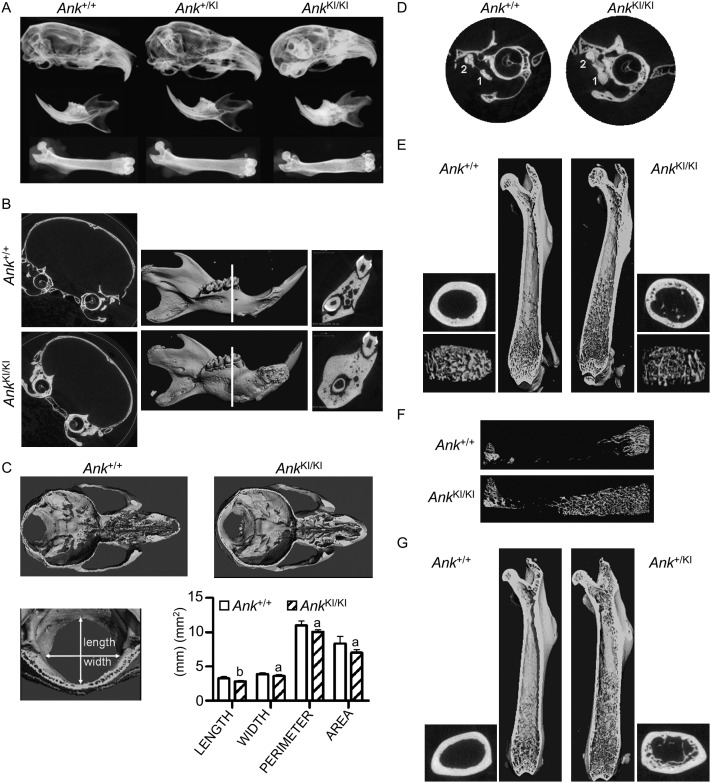
FIG. 2.
CMD-like phenotype in Ank KI/KI mice. (A) Representative radiographs of skulls, mandibles, and femurs from 6-mo-old Ank +/+, Ank +/KI, and Ank KI/KI male mice. (B) μCT images of frontal plane through skulls and 3D reconstruction of mandibles from 3-mo-old Ank +/+ and Ank KI/KI male mice. White line indicates sagittal plane through furcation of first mandibular molar. (C) Internal, dorsal view of cranial floor and nasal cavities from horizontal plane of superior semicircular duct and the cribriform plate of ethmoid. Histogram shows dimensions of foramen magnum of Ank +/+ and Ank KI/KI littermates. (D) 2D μCT images of tympanic bulla from frontal plane through cochlea showing fusion of malleus (1) and incus (2). (E) Internal view of Ank +/+ and Ank KI/KI femurs. 3D reconstructions of trabeculation in metaphysis and cross-sectional slices of cortical bone in diaphysis. (F) 3D μCT images of total trabecular bone in femurs from Ank +/+ and Ank KI/KI mice at 10 wk of age. (G) Internal view of femurs of 12-mo-old Ank +/+ and Ank +/KI mice. 3D reconstructions of trabeculation in metaphysis and cross-sectional slices of cortical bone in diaphysis.
We examined BMC and BMD of Ank +/+ and Ank KI/KI mice by DXA. Our results showed that Ank KI/KI mice had increased BMC and BMD in skulls and mandibles, whereas measurements of cortical bones in diaphyses showed increased BMC but normal BMD (Table 1). The same cortical diaphyses were subjected to ash weight determination. Unexpectedly, ash content in the Ank KI/KI group (67.487 ± 1.255%, n = 11) was significantly lower compared with Ank +/+ mice (69.731 ± 1.398%, n = 11; p < 0.01). This finding was consistent with μCT, which showed a 5% reduction of cortical tissue density in Ank KI/KI mice (Table 2).
Table 1.
DXA Measurement of Ank +/+ and Ank KI/KI Mice
|
Ank+/+ |
AnkKI/KI |
|||||
| Skull (n = 8) | Mandible (n = 8) | Femur (n = 11) | Skull (n = 6) | Mandible (n = 6) | Femur (n = 11) | |
| BMC (g) | 0.176 ± 0.011 | 0.028 ± 0.002 | 0.008 ± 0.002 | 0.251 ± 0.008* | 0.039 ± 0.004* | 0.011 ± 0.001† |
| BMD (g/cm2) | 0.087 ± 0.004 | 0.063 ± 0.002 | 0.058 ± 0.007 | 0.123 ± 0.003* | 0.078 ± 0.005* | 0.060 ± 0.003 |
Measurements of mice at 10 wk of age were determined by DXA. Data are mean ± SD for groups of 6–11 mice.
* p < 0.01.
† p < 0.05.
Table 2.
μCT Analysis of 3-mo-old Ank +/+ and Ank KI/KI Male Mice
| Ank+/+(n = 5) | AnkKI/KI (n = 7) | |
| Calvariae | ||
| Cortical width (mm) | 0.14 ± 0.02 | 0.17 ± 0.01* |
| BM/TA (%) | 16.9 ± 2.2 | 16.1 ± 7.3 |
| BA/TA (%) | 83.1 ± 2.2 | 83.9 ± 7.3 |
| Mandibles | ||
| BVF (%) | 77 ± 1.9 | 88.7 ± 2.8† |
| Dentin density | 1493 ± 62 | 1311 ± 106† |
| Femurs trabecular bone (metaphyses) | ||
| BVF (%) | 30.2 ± 7.1 | 10.7 ± 1.4† |
| Trabecular number(N/mm) | 5.94 ± 0.48 | 5.02 ± 0.38† |
| Trabecular thickness (μm) | 66 ± 14 | 45 ± 5† |
| Trabecular spacing (μm) | 160 ± 20 | 196 ± 17‡ |
| Femurs cortical bone (diaphyses) | ||
| Subperiosteal area (mm2) | 1.94 ± 0.241 | 2.35 ± 0.29‡ |
| Subendosteal area (mm2) | 0.79 ± 0.14 | 1.22 ± 0.18† |
| Cortical porosity (%) | 3.4 ± 0.6 | 5.7 ± 2.1† |
| Tissue density (mg/cm3 HA) | 1502 ± 19 | 1435 ± 18† |
| Femurs whole trabecular bone | ||
| Bone volume (mm3) | 1.005 ± 0.22 | 1.280 ± 0.33 |
| SMI | 1.8 ± 0.4 | 2.9 ± 0.3† |
BM, calvarial total bone marrow space; BA, calvarial bone area; TA, calvarial total area; BV, bone volume; TV, total volume; BVF, BV/TV.
Data are mean ± SD.
* p = 0.0505 for cortical width.
† p < 0.01.
‡ p < 0.05.
To better characterize the bone phenotype, we studied the skeleton of 3-mo-old Ank +/+ and Ank KI/KI mice by μCT. Craniofacial bones of Ank KI/KI mice displayed hyperostosis, especially in the cranial base and the mandibles (Fig. 2B). Ank KI/KI mice showed a trend for increase in calvarial width (p = 0.0505) and a significant increase in bone mass of mandibles (Table 2). Hyperostosis of the cranial base in Ank KI/KI mice was accompanied by narrowing of cranial neural foramina and obliteration of nasal sinuses (Fig. 2C). The size of the foramen magnum was significantly reduced in all dimensions (Fig. 2C). Moreover, 2D images showed fusion of incus and malleus in Ank KI/KI mice, suggesting impaired hearing (Fig. 2D). Another characteristic of CMD patients is flaring metaphysis with increased radiolucency. Ank KI/KI mice exhibited abnormal shape of femurs with increased diameter of cortical bone and undertrabeculated metaphyses (Fig. 2E; Table 2). These metaphyseal trabeculae were reduced, both in thickness and numbers, compared with Ank +/+ mice. Furthermore, Ank KI/KI mice showed increased porosity of cortical bones. Whereas Ank +/+ mice had most trabecular bone restricted to metaphyses, Ank KI/KI mice extended trabecular bones into diaphyses (Fig. 2F). Although significantly decreased trabeculation was observed in metaphyses of Ank KI/KI mice, the total volume of trabecular bone in femurs of Ank +/+ and Ank KI/KI mice showed no significant difference. However, the structure model index (SMI) for Ank KI/KI mice suggested that trabecular bones are more rod-shaped in contrast to more plate-like in Ank +/+ mice (Table 2). To our knowledge, distribution of trabecular bone in diaphyses of CMD patients has not been studied.
Skeletal phenotype of Ank+/KI mice
Heterozygous Ank+/KI mice were phenotypically similar to wildtype mice but exhibited a mild CMD-like phenotype as they aged. When comparing 1-yr-old Ank +/+ (n = 6) and Ank +/KI (n = 7) male mice by μCT, we found that Ank +/KI mice developed a femoral shape comparable to Ank KI/KI mice with extensive trabecular bone in diaphyses (Fig. 2G). However, we did not detect a significant difference in hyperostotic phenotypes of craniofacial bones because of large variability (data not shown). Variable expressivity has been observed in autosomal dominant CMD patients as well.(29)
Biochemical analysis of AnkKI/KI mice
CMD patients present with elevated serum ALP, increased TRACP, and normal to slightly/transiently elevated PTH levels.(3,6,13,30) In sera of 10-wk-old fasted Ank KI/KI mice, we detected increases in ALP, a nonspecific bone marker, and TRACP5b, a marker of osteoclast numbers (Table 3). To further study osteoblast and osteoclast activities, we measured P1NP, a measure of bone formation, and CTX, a marker of osteoclast activity in age- and sex-matched samples from fasted animals. We detected a wide range of P1NP (60.5–137.15 ng/ml) and CTX (18.20–32.49 ng/ml) in Ank +/+ mice. Most P1NP and CTX values in Ank KI/KI mice were in the upper end of these intervals or above, resulting in statistical significance (Table 3). These results, taken together, suggest increased bone turnover in Ank KI/KI mice.
Table 3.
Serum Parameters in 10-wk-old Ank +/+ and Ank KI/KI Male Mice
| Serum levels | Ank+/+ | AnkKI/KI |
| ALP (μM/15ul) (Ank +/+n = 6, Ank KI/KI n = 7) | 17.16 ± 3.52 | 24.37 ± 5.78* |
| TRACP5b (U/liter) (Ank +/+n = 7, Ank KI/KI n = 7) | 7.15 ± 0.62 | 10.83 ± 1.89† |
| P1NP (ng/ml) (Ank +/+n = 14, Ank KI/KI n = 14) | 88.82 ± 25.24 | 108.57 ± 26.26* |
| CTX (ng/ml) (Ank +/+n = 18, Ank KI/KI n = 18) | 24.10 ± 4.49 | 30.49 ± 6.39† |
Fasting serum was used for all measurement. Data are mean ± SD.
* p < 0.05.
† p < 0.01.
Bone histology and histomorphometry of AnkKI/KI mice
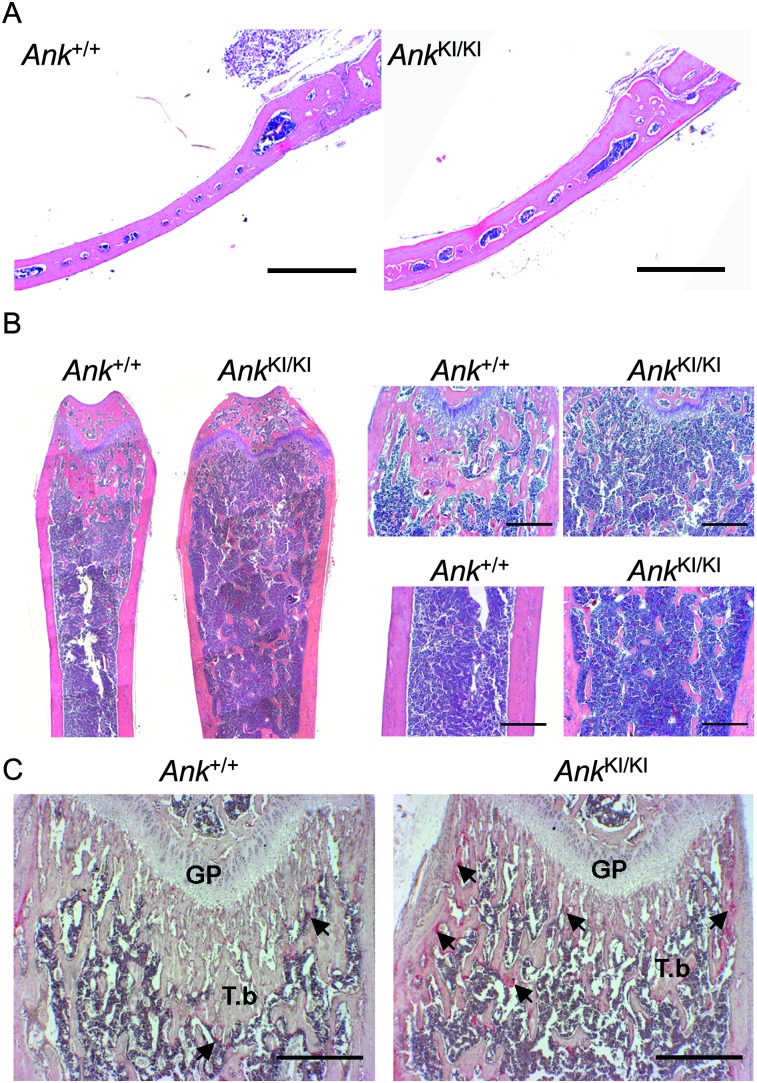
We performed bone histology and histomorphometry on groups of 10-wk-old Ank +/+ and Ank KI/KI male mice. Histology sections confirmed that Ank KI/KI calvariae were thicker than those of Ank +/+ mice (Fig. 3A). Ank KI/KI femurs exhibited widened growth plates, reduced trabeculation in metaphyses, while retaining trabecular bone in diaphyses (Fig. 3B). Static histomorphometry showed that Ank KI/KI mice exhibited a small but significant increase in osteoblast surface (1.15-fold) and osteoclast surface (2-fold) in femurs and an increase in osteoclast surface (1.8-fold) in calvariae (Table 4). Increased numbers of TRACP+ cells in the metaphysis, chondro-osseous junction, and the endosteum of femoral epiphysis and metaphysis were already observed in 1-mo-old Ank KI/KI mice (Fig. 3C).
FIG. 3.
Histology of Ank +/+ and Ank KI/KI male mice. (A) Calvariae from 10-wk-old mice (H&E). (B) H&E staining of 10-wk-old mice: femurs (left panel), metaphyses (top right), and diaphyses (bottom right). (C) TRACP staining of femurs from 4-wk-old mice. Arrows indicate TRACP+ cells. GP, growth plate; T.b, trabecular bone. Scale bar = 500 μm.
Table 4.
Static Histomorphometry of 10-wk-old Ank +/+ and Ank KI/KI Male Mice
| Ank+/+(n = 5) | AnkKI/KI (n = 5) | |
| Calvariae | ||
| Oc.Pm (mm) | 0.44 ± 0.22 | 0.79 ± 0.07* |
| Oc.Pm/B.Pm (%) | 10.15 ± 4.9 | 18.13 ± 4.51† |
| Femoral metaphyses | Ank+/+ (n = 8) | AnkKI/KI (n = 7) |
| BVF (%) | 9.2 ± 2.7 | 5.0 ± 2.2* |
| Trabecular thickness (μm) | 33.1 ± 6.1 | 21.1 ± 4.5* |
| Ob.S/BS (%) | 22.2 ± 2.7 | 25.4 ± 2.5† |
| Oc.S/BS (%) | 3.4 ± 2 | 7.1 ± 2.5† |
| N.Oc/B.Pm (N/mm) | 2.0 ± 1.1 | 4.1 ± 1.1* |
| N.Oc/T.Ar (N/mm2) | 10.5 ± 5.7 | 20.8 ± 6.1* |
Data are mean ± SD.
* p < 0.01.
† p < 0.05.
Oc.Pm, osteoclast surface; Oc.Pm/B.Pm, osteoclast surface measured on the total trabecular bone surface; Ob.S/BS, osteoblast surface (osteoblast surface/bone surface); Oc.S/BS, osteoclast surface (osteoclast surface/bone surface); N.Oc/B.Pm, osteoclast number over the total trabecular bone surface; N.Oc/T.Ar, number of osteoclasts normalized by total area measured.
For dynamic histomorphometry, we labeled mice with calcein and xylenol orange. Ank KI/KI mice showed a decreasing trend in mineral apposition rate in femoral metaphyses (Ank +/+: 1.83 ± 0.24 μm/d, n = 6; Ank KI/KI: 1.63 ± 0.08 μm/d, n = 10; p = 0.1) and calvariae (Ank +/+: 1.34 ± 0.28 μm/d, n = 7; Ank KI/KI: 1.07 ± 0.25 μm/d, n = 9; p = 0.06). However, no significant difference in bone formation rate of Ank +/+ and Ank KI/KI littermates was observed in femoral metaphyses (Ank +/+: 0.66 ± 0.18 μm3/μm2/d; Ank KI/KI: 0.64 ± 0.16 μm3/μm2/d; p = 0.82) or in calvariae (Ank +/+: 0.547 ± 0.239 μm3/μm2/d; Ank KI/KI: 0.529 ± 0.257 μm3/μm2/d; p = 0.88).
In vitro osteoclastogenesis
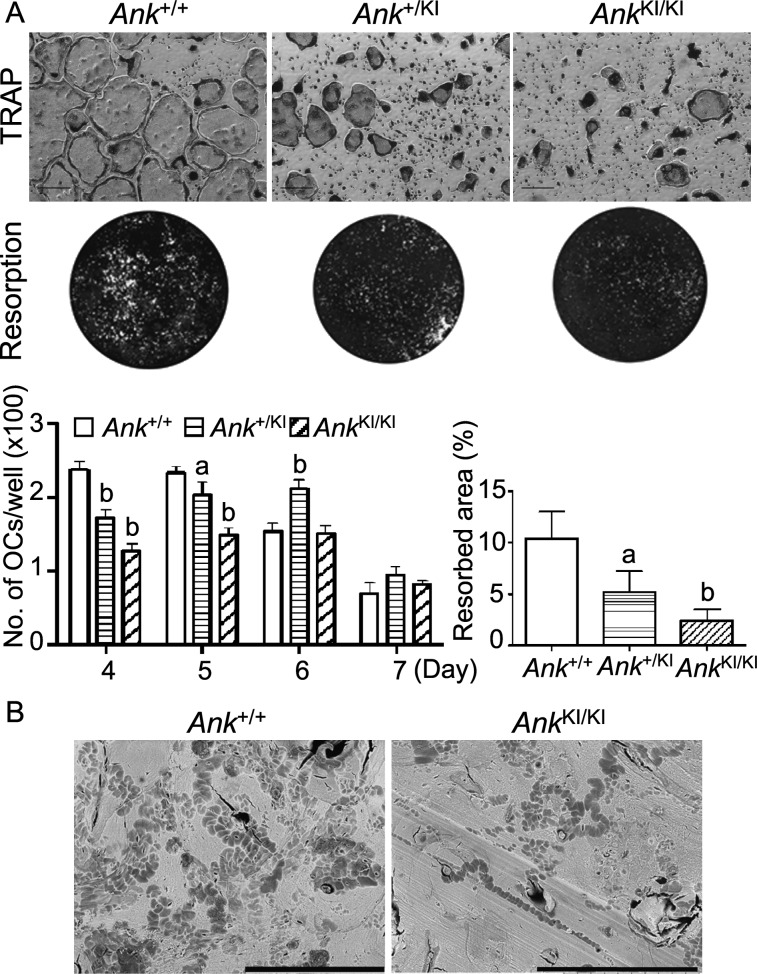
Yamamoto et al.(13) described dysfunctional osteoclastogenesis in bone marrow–derived cultures of a 3-yr-old CMD patient who had increased serum TRACP and elevated urinary hydroxyproline excretion. We examined osteoclastogenesis using Ank +/+ and Ank KI/KI bone marrow–derived macrophage (BMM) cultures. BMMs were cultured in the presence of M-CSF and RANKL for 4, 5, 6, and 7 days. Cells were TRACP stained, and TRACP+ cells with three or more nuclei were considered osteoclasts. During in vitro osteoclast differentiation, Ank KI/KI BMMs formed significantly fewer and smaller osteoclasts (Fig. 4A). Furthermore, Ank KI/KI cells showed marked reduction of mineral resorption on calcium phosphate–coated slides (Fig. 4A). This result was confirmed by resorption pit assays on bone chips (Fig. 4B; Ank +/+ = 41.6 ± 5.83%, Ank KI/KI = 17.6 ± 3.10%, p < 0.01). Ank +/KI BMMs showed significant decreased osteoclast formation and resorption, although less severe than Ank KI/KI cultures. We conclude that the Ank Phe377del mutation leads to decreased in vitro osteoclastogenesis, consistent with the finding in a CMD case report.(13)
FIG. 4.
In vitro osteoclast assays. (A) Representative images of TRACP staining at day 5 and of resorption of osteologic slides in BMM cultures. Histograms show number of mature osteoclasts per well and percentile of the resorbed area., Analysis of TRACP staining was performed by two-way ANOVA followed by Bonferroni test compared with the Ank +/+ group for each time point. Resorption assay was evaluated by one-way ANOVA with Tukey's multiple-comparison test. a p < 0.05; b p < 0.01. Scale bar = 200 μm. (B) Representative images of resorption pit assay on bone chips. Scale bar = 300 μm.
Bone matrix analysis of AnkKI/KI mice
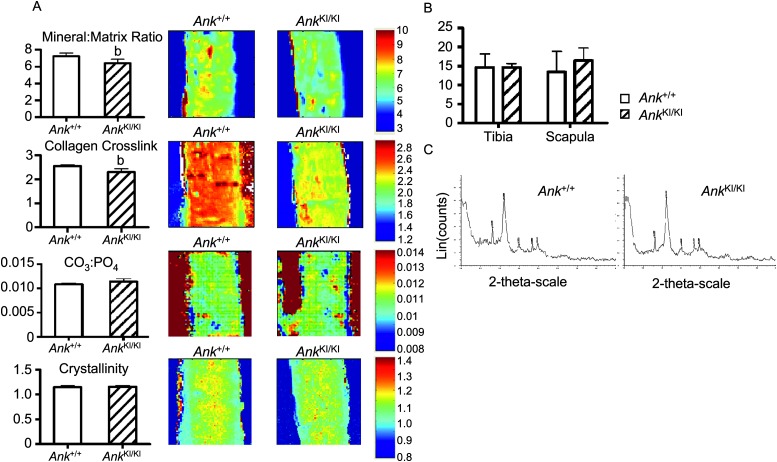
To evaluate the effects of the Ank mutation on bone quality, we examined bone matrix on femoral sections by FTIR analysis. Mineral content (mineral-to-matrix ratio), which corresponds to ash weight of bone, was significantly decreased in the central cortical bone of Ank KI/KI femurs (Fig. 5A). Collagen maturity, which is determined by the relative ratio of nonreducible and reducible collagen cross-links, was significantly reduced in Ank KI/KI mice compared with Ank +/+ mice (Fig. 5A). The carbonate-to-phosphate ratio (replacement of phosphate by carbonate groups) and crystallinity (which corresponds to the crystal size and perfection determined by X-ray diffraction) remained unaffected in cortical bones of Ank KI/KI mice (Fig. 5A).
FIG. 5.
FTIR and X-ray diffraction analysis of bones from 10-wk-old Ank +/+ and Ank KI/KI male mice. (A) Mineral:matrix ratio; collagen cross-linking; carbonate:phosphate ratio; crystallinity of central cortical bones from 10-wk-old Ank +/+ and Ank KI/KI femurs. Numerical scales represent the range of intensity ratios applied for each parameter. Histograms for individual images are shown on the left. (B) Analysis of crystal size in bone powder from tibias and scapulae by X-ray diffraction. (C) Crystalline phases in bone powder identified by wide-angle X-ray diffraction. Data are mean ± SD. Statistical significance by Student's t-test (b p < 0.01).
To determine the size and type of crystals, we used wide-angle X-ray diffraction. In agreement with FTIR results, crystal size and perfection in bone powder from tibia and scapula were comparable between Ank +/+ and Ank KI/KI mice (Fig. 5B). Furthermore, we detected hydroxyapatite, not CPPD, crystals in both groups (Fig. 5C).
Comparison of skeletal phenotypes of AnkKI/KI and Ankank/ank mice
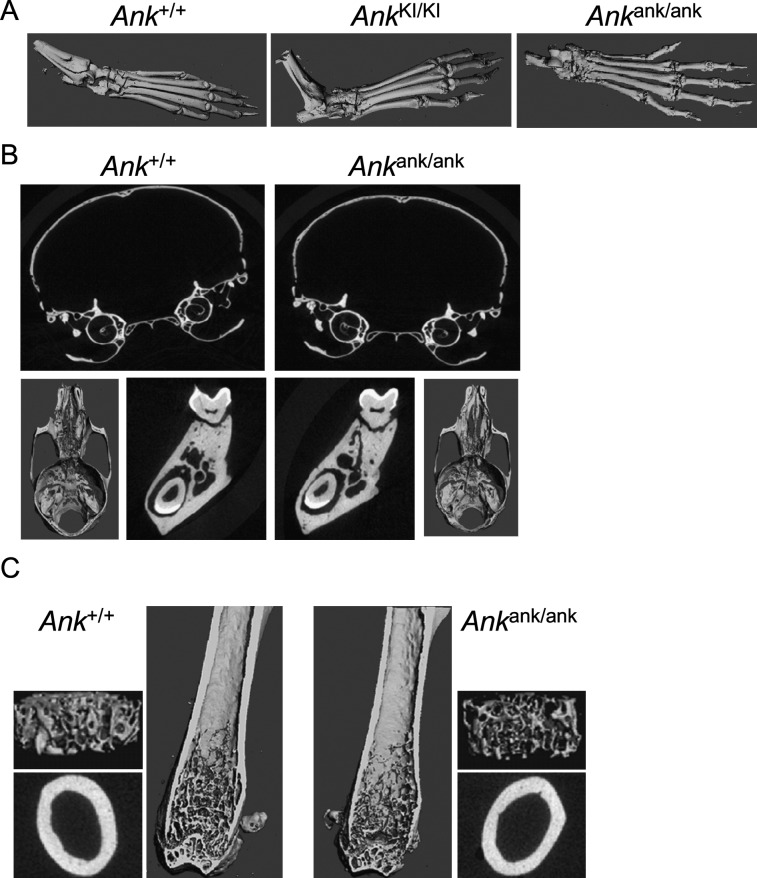
We compared joint and bone phenotypes of Ank KI/KI and Ank ank/ank mice. Both Ank KI/KI and Ank ank/ank mice developed joint stiffness and could not grab cage bars starting at ages of 4–5 wk. In Ank ank/ank joints, we observed excessive bony deposits, which appeared more severe than in Ank KI/KI mice (Fig. 6A). Ank ank/ank mice developed less trabeculation in metaphyses; however, in contrast to Ank KI/KI mice, they failed to present mandibular hyperostosis, narrowed nasal sinuses, flared metaphyses, or trabecular bone in diaphyses (Figs. 6B and 6C). Those findings suggest that the CMD-like phenotype in Ank KI/KI mice is distinct and is not simply caused by a loss of PPi transport function of ANK.
FIG. 6.
Skeletal phenotype of 3-mo-old Ank ank/ank male mice. (A) 3D μCT images comparing hind feet of Ank +/+, Ank KI/KI, and Ank ank/ank animals. (B) μCT images of skulls, cranial and nasal cavities, and mandibles. (C) Internal view of Ank +/+ and Ank ank/ank femurs. 3D reconstructions of trabeculation in metaphysis and cross-sectional slices of cortical bone in diaphysis.
DISCUSSION
Although Ank KI/KI mice exhibit many features of CMD, they differ from human CMD in two aspects. Whereas the Phe377del mutation in humans leads to CMD in an AD trait, most heterozygous mice appear phenotypically closer to wildtype littermates but develop an intermediate phenotype with variable expressivity as they age. It is not unusual that the expression of an AD human disease gene results in severe phenotypes only in homozygote mice.(31–33) Differences between the disease characteristics of autosomal dominant CMD in humans and Ank +/KI mice may be because of species-specific phenotypic thresholds and lifespan. Joint stiffness of elbows, knees, paws, and vertebrae in Ank KI/KI, Ank ank/ank, and Ank null/null mice has not been reported in CMD patients. However, we have not found patients who are homozygous or compound heterozygous for CMD mutations.
Ank ank/ank mice display more severe ectopic mineralization in joints and lack certain CMD-specific features in comparison with Ank KI/KI mice. The joint phenotype in Ank loss-of-function models is caused by ectopic deposition of HA from reduced extracellular PPi, which is an inhibitor of mineralization. It is conceivable that the CMD mutation does not fully inhibit PPi transport or that extracellular PPi synthesis by phosphodiesterases in Ank KI/KI mice is higher than in Ank ank/ank or Ank null/null mice. The unique CMD-like phenotype develops before the onset of joint stiffness in Ank KI/KI mice. In 1-wk-old Ank KI/KI mice, we observed a massive mandible, flaring metaphyses, and less trabecular bone but more trabeculation in diaphyses (data not shown). These skeletal comparisons suggest that Ank KI/KI mice represent a better model for CMD and loss of PPi transporter function is not the sole cause for CMD. Based on the clustering of CMD mutations, we hypothesize that mutant ANK potentially alters protein–protein interactions and/or intracellular signal transduction, resulting in the unique CMD phenotype.
Biochemical serum markers can be used as measures for osteoblast and osteoclast activities.(34) Total serum ALP is widely used as a marker for bone metabolism, although it is not bone specific. Data from two of our CMD patients showed elevated levels for both bone- and liver-specific ALP (data not shown). Increased TRACP5b is consistent with increased osteoclast numbers as shown by static histomorphometry in Ank KI/KI mice. Serum levels of P1NP and CTX, measures of bone formation and bone resorption, have not been reported in CMD patients. We interpret the increased serum P1NP and CTX in Ank KI/KI mice as increased bone turnover. Decreased trabecular thickness and increased cortical porosity in these mice may be results of increased bone remodeling activity.
We observed hypomineralized bone matrix in Ank KI/KI mice by ash weight, μCT, and FTIR. Reduced BMD has also been reported in one CMD patient.(1) Conversely, DXA showed increased BMC in skulls, mandibles, and femurs of Ank KI/KI mice. Although DXA is a clinically used noninvasive method to assess bone mass, it only measures density per area and not by volume. Hyperostosis and changes in bone shape could result in overestimation of BMC and BMD and should be taken into consideration when using DXA.(35) We suggest that hyperostosis cannot be equated with sclerosis as is done in some clinical reports of CMD.
FTIR analysis provides information about chemical properties of bone. The mineral phase in the femoral cortex was homogeneously distributed; however, the mineral to matrix ratio and collagen maturity were decreased in Ank KI/KI specimen. Mineralization defects can be caused by improper collagen cross-links, enhanced bone matrix synthesis, or inappropriate mineralization of the secreted matrix.(36) Overall bone strength can be attributed to both structural and material components. Therefore, it is possible that increased bone diameters with club shape and abnormal distribution of trabecular bones in diaphyses is an attempt to compensate for poor bone quality and provide improved structural properties in Ank KI/KI mice.
A tight balance between the levels of extracellular phosphate (Pi) and pyrophosphate (PPi) is required to control mineralization. Extracellular PPi (ePPi) acts as a potent inhibitor of HA mineralization and exhibits a bimodal effect on crystal formation. Low ePPi leads to excess HA formation, whereas supersaturation of ePPi promotes CPPD crystal deposition.(37) Whether mutant ANK causes altered PPi transport into extracellular matrix has not been tested in CMD patients. However, an in vitro assay showed that overexpression of a BAC-Ank G389R construct, one of the identified CMD-causing mutations, in oocytes leads to significantly reduced PPi uptake.(25) We determined by X-ray diffraction that abnormal mineral crystals from bones of Ank KI/KI mice consist of HA and not CPPD, which indicated that Ank KI/KI mice do not exhibit excessive ePPi levels. Whether the Phe377del mutation in ANK results in less PPi transport to extracellular matrix and whether other PPi regulators, such as ectonucleotide pyrophosphatase phosphodiesterases or tissue-nonspecific ALP, can compensate for a decrease of ANK activity in this mouse model is a subject of future studies.
To date, two hypotheses have been proposed for the pathogenesis of CMD: reduced bone resorption(12,13) and increased bone formation secondary to increased bone turnover.(3) Both hypotheses, however, cannot fully explain hyperostosis of craniofacial bones and undertrabeculated metaphyses of long bones in CMD. A previous study and our data showed decreased osteoclastogenesis in vitro, whereas elevated serum TRACP5b was detected in a CMD patient(13) and in Ank KI/KI mice. Therefore, increased cell numbers could possibly be a compensation of reduced osteoclast function in CMD patients and Ank KI/KI mice. Our results suggested that Ank KI/KI mice have increased bone turnover, which may lead to either increased or decreased bone mass. We propose that metaphyses in Ank KI/KI mice develop imbalanced bone remodeling in favor of resorption, whereas in cranial bones, bone formation overweighs bone resorption. Cranial vault and jawbones are formed by intramembranous ossification, whereas long bones develop through endochondral bone formation, a process involving chondrogenesis.(38,39) Calcified cartilage septa are required for osteoblasts to lay down primary trabecular bone.(40) Because Ank is expressed in chondrocytes, it is possible that a primary chondrocyte defect contributes to the long bone phenotype. The increase in osteoclast numbers, the proximity to the growth plate, and an environment rich in bone marrow (a source of osteoclast progenitors) may result in net bone loss in the metaphysis. Transverse widening of long bone growth plates is regulated by appositional growth at the groove of Ranvier.(41,42) Bone bark, a component of the groove of Ranvier, is responsible for widening of the cortical portion of bone, and endosteal and periosteal osteoclast activity in this region is needed for contouring bone shape and width.(42) The abnormal metaphyseal bone shape may be a result of increased endosteal bone resorption in the region described as bone bark and of reduced periosteal resorption outside of the conductive metaphyseal environment. On the other hand, in craniofacial bones, there is less bone marrow present and, despite increased numbers of osteoclasts, their overall activity may be reduced.
In summary, we successfully generated the first mouse model for CMD, which will allow the study of CMD pathogenesis at cellular and molecular levels. Our results imply a previously unappreciated complexity of the CMD phenotype involving both osteoblasts and osteoclasts. We expect that additional studies of this model will help to address the mechanisms of CMD-causing ANK mutations and potentially lead to therapeutic approaches for CMD.
ACKNOWLEDGMENTS
We are indebted to members of the UCHC Bone Group at UCHC for helpful discussions. The authors thank the μCT and Histomorphometry facilities at UCHC for support. The project was supported by Grants AR49539 (NIAMS) to E.J.R. and DE007302 to C.J.W.
Footnotes
The authors state that they have no conflicts of interest.
REFERENCES
- 1.Elcioglu N, Hall CM. Temporal aspects in craniometaphyseal dysplasia: Autosomal recessive type. Am J Med Genet. 1998;76:245–251. doi: 10.1002/(sici)1096-8628(19980319)76:3<245::aid-ajmg8>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 2.Ramseyer LT, Leonard JC, Stacy TM. Bone scan findings in craniometaphyseal dysplasia. Clin Nucl Med. 1993;18:137–139. doi: 10.1097/00003072-199302000-00011. [DOI] [PubMed] [Google Scholar]
- 3.Fanconi S, Fischer JA, Wieland P, Giedion A, Boltshauser E, Olah AJ, Landolt AM, Prader A. Craniometaphyseal dysplasia with increased bone turnover and secondary hyperparathyroidism: Therapeutic effect of calcitonin. J Pediatr. 1988;112:587–591. doi: 10.1016/s0022-3476(88)80176-8. [DOI] [PubMed] [Google Scholar]
- 4.Haverkamp F, Emons D, Straehler-Pohl HJ, Zerres K. Craniometaphyseal dysplasia as a rare cause of a severe neonatal nasal obstruction. Int J Pediatr Otorhinolaryngol. 1996;34:159–164. doi: 10.1016/0165-5876(95)01244-3. [DOI] [PubMed] [Google Scholar]
- 5.Key LL, Jr, Volberg F, Baron R, Anast CS. Treatment of craniometaphyseal dysplasia with calcitriol. J Pediatr. 1988;112:583–587. doi: 10.1016/s0022-3476(88)80175-6. [DOI] [PubMed] [Google Scholar]
- 6.Cheung VG, Boechat MI, Barrett CT. Bilateral choanal narrowing as a presentation of craniometaphyseal dysplasia. J Perinatol. 1997;17:241–243. [PubMed] [Google Scholar]
- 7.Schwahn B, Schaper J, Herkenrath P, Michel O, Schoenau E. Autosomal-dominante kraniometaphysaere dysplasie. Monatsschr Kinderheilkd. 1996;144:1073–1077. [Google Scholar]
- 8.Langer LO, Jr, Brill PW, Afshani E, Williams CA, Thomas IT, Frias JL. Radiographic features of craniometadiaphyseal dysplasia, wormian bone type. Skeletal Radiol. 1991;20:37–41. doi: 10.1007/BF00243719. [DOI] [PubMed] [Google Scholar]
- 9.Richards A, Brain C, Dillon MJ, Bailey CM. Craniometaphyseal and craniodiaphyseal dysplasia, head and neck manifestations and management. J Laryngol Otol. 1996;110:328–338. doi: 10.1017/s0022215100133560. [DOI] [PubMed] [Google Scholar]
- 10.Halliday J. A rare case of bone dystrophy. Br J Surg. 1949;37:52–63. doi: 10.1002/bjs.18003714509. [DOI] [PubMed] [Google Scholar]
- 11.Jackson WPU, Albright F, Drewery G, Hanelin J, Rubin ML. Metaphyseal dysplasia, epiphyseal dysplasia, diaphyseal dysplasia and related conditions. Arch Intern Med. 1954;94:871–885. doi: 10.1001/archinte.1954.00250060005001. [DOI] [PubMed] [Google Scholar]
- 12.Millard DR, Jr, Maisels DO, Batstone JH, Yates BW. Craniofacial surgery in craniometaphyseal dysplasia. Am J Surg. 1967;113:615–621. doi: 10.1016/0002-9610(67)90307-8. [DOI] [PubMed] [Google Scholar]
- 13.Yamamoto T, Kurihara N, Yamaoka K, Ozono K, Okada M, Yamamoto K, Matsumoto S, Michigami T, Ono J, Okada S. Bone marrow-derived osteoclast-like cells from a patient with craniometaphyseal dysplasia lack expression of osteoclast-reactive vacuolar proton pump. J Clin Invest. 1993;91:362–367. doi: 10.1172/JCI116194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gorlin RJ, Anderson RC, Blaw M. Multiple lentigenes syndrome. Am J Dis Child. 1969;117:652–662. doi: 10.1001/archpedi.1969.02100030654006. [DOI] [PubMed] [Google Scholar]
- 15.Reichenberger E, Tiziani V, Watanabe S, Park L, Ueki Y, Santanna C, Baur ST, Shiang R, Grange DK, Beighton P, Gardner J, Hamersma H, Sellars S, Ramesar R, Lidral AC, Sommer A, Raposo do Amaral CM, Gorlin RJ, Mulliken JB, Olsen BR. Autosomal dominant craniometaphyseal dysplasia is caused by mutations in the transmembrane protein ANK. Am J Hum Genet. 2001;68:1321–1326. doi: 10.1086/320612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nurnberg P, Thiele H, Chandler D, Hohne W, Cunningham ML, Ritter H, Leschik G, Uhlmann K, Mischung C, Harrop K, Goldblatt J, Borochowitz ZU, Kotzot D, Westermann F, Mundlos S, Braun HS, Laing N, Tinschert S. Heterozygous mutations in ANKH, the human ortholog of the mouse progressive ankylosis gene, result in craniometaphyseal dysplasia. Nat Genet. 2001;28:37–41. doi: 10.1038/ng0501-37. [DOI] [PubMed] [Google Scholar]
- 17.Pendleton A, Johnson MD, Hughes A, Gurley KA, Ho AM, Doherty M, Dixey J, Gillet P, Loeuille D, McGrath R, Reginato A, Shiang R, Wright G, Netter P, Williams C, Kingsley DM. Mutations in ANKH cause chondrocalcinosis. Am J Hum Genet. 2002;71:933–940. doi: 10.1086/343054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williams CJ, Zhang Y, Timms A, Bonavita G, Caeiro F, Broxholme J, Cuthbertson J, Jones Y, Marchegiani R, Reginato A, Russell RG, Wordsworth BP, Carr AJ, Brown MA. Autosomal dominant familial calcium pyrophosphate dihydrate deposition disease is caused by mutation in the transmembrane protein ANKH. Am J Hum Genet. 2002;71:985–991. doi: 10.1086/343053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zaka R, Stokes D, Dion AS, Kusnierz A, Han F, Williams CJ. P5L mutation in Ank results in an increase in extracellular inorganic pyrophosphate during proliferation and nonmineralizing hypertrophy in stably transduced ATDC5 cells. Arthritis Res Ther. 2006;8:R164. doi: 10.1186/ar2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ho AM, Johnson MD, Kingsley DM. Role of the mouse ank gene in control of tissue calcification and arthritis. Science. 2000;289:265–270. doi: 10.1126/science.289.5477.265. [DOI] [PubMed] [Google Scholar]
- 21.Carr G, Sayer JA, Simmons NL. Expression and localisation of the pyrophosphate transporter, ANK, in murine kidney cells. Cell Physiol Biochem. 2007;20:507–516. doi: 10.1159/000107534. [DOI] [PubMed] [Google Scholar]
- 22.Gurley KA, Chen H, Guenther C, Nguyen ET, Rountree RB, Schoor M, Kingsley DM. Mineral formation in joints caused by complete or joint-specific loss of ANK function. J Bone Miner Res. 2006;21:1238–1247. doi: 10.1359/jbmr.060515. [DOI] [PubMed] [Google Scholar]
- 23.Sweet HO, Green MC. Progressive ankylosis, a new skeletal mutation in the mouse. J Hered. 1981;72:87–93. doi: 10.1093/oxfordjournals.jhered.a109459. [DOI] [PubMed] [Google Scholar]
- 24.Hakim FT, Cranley R, Brown KS, Eanes ED, Harne L, Oppenheim JJ. Hereditary joint disorder in progressive ankylosis (ank/ank) mice. I. Association of calcium hydroxyapatite deposition with inflammatory arthropathy. Arthritis Rheum. 1984;27:1411–1420. doi: 10.1002/art.1780271212. [DOI] [PubMed] [Google Scholar]
- 25.Gurley KA, Reimer RJ, Kingsley DM. Biochemical and genetic analysis of ANK in arthritis and bone disease. Am J Hum Genet. 2006;79:1017–1029. doi: 10.1086/509881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hausamen TUH, Rick R, Gross W, W Optimal conditions for the determination of serum alkaline phosphatase by a new kinetic method. Clin Chim Acta. 1967;15:241–245. [Google Scholar]
- 27.Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR. Bone histomorphometry: Standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 1987;2:595–610. doi: 10.1002/jbmr.5650020617. [DOI] [PubMed] [Google Scholar]
- 28.Gorlin RJ, Cohen MM, Jr, Hennekam RCM. 4th ed. New York, NY, USA: Oxford Press; 2001. Syndromes of the Head and Neck. [Google Scholar]
- 29.Carnevale A, Grether P, del Castillo V, Takenaga R, Orzechowski A. Autosomal dominant craniometaphyseal dysplasia. Clinical variability. Clin Genet. 1983;23:17–22. doi: 10.1111/j.1399-0004.1983.tb00431.x. [DOI] [PubMed] [Google Scholar]
- 30.Sheppard WM, Shprintzen RJ, Tatum SA, Woods CI. Craniometaphyseal dysplasia: A case report and review of medical and surgical management. Int J Pediatr Otorhinolaryngol. 2003;67:71–77. doi: 10.1016/s0165-5876(02)00289-6. [DOI] [PubMed] [Google Scholar]
- 31.Chipman SD, Sweet HO, McBride DJ, Jr, Davisson MT, Marks SC, Jr, Shuldiner AR, Wenstrup RJ, Rowe DW, Shapiro JR. Defective pro alpha 2(I) collagen synthesis in a recessive mutation in mice: A model of human osteogenesis imperfecta. Proc Natl Acad Sci USA. 1993;90:1701–1705. doi: 10.1073/pnas.90.5.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ueki Y, Lin CY, Senoo M, Ebihara T, Agata N, Onji M, Saheki Y, Kawai T, Mukherjee PM, Reichenberger E, Olsen BR. Increased myeloid cell responses to M-CSF and RANKL cause bone loss and inflammation in SH3BP2 “cherubism” mice. Cell. 2007;128:71–83. doi: 10.1016/j.cell.2006.10.047. [DOI] [PubMed] [Google Scholar]
- 33.Liao BY, Zhang J. Null mutations in human and mouse orthologs frequently result in different phenotypes. Proc Natl Acad Sci USA. 2008;105:6987–6992. doi: 10.1073/pnas.0800387105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seibel MJ. Molecular markers of bone turnover: Biochemical, technical and analytical aspects. Osteoporos Int. 2000;11(Suppl 6):S18–S29. doi: 10.1007/s001980070003. [DOI] [PubMed] [Google Scholar]
- 35.Ott SM, O'Hanlan M, Lipkin EW, Newell-Morris L. Evaluation of vertebral volumetric vs. areal bone mineral density during growth. Bone. 1997;20:553–556. doi: 10.1016/s8756-3282(97)00057-4. [DOI] [PubMed] [Google Scholar]
- 36.Knott L, Bailey AJ. Collagen cross-links in mineralizing tissues: A review of their chemistry, function, and clinical relevance. Bone. 1998;22:181–187. doi: 10.1016/s8756-3282(97)00279-2. [DOI] [PubMed] [Google Scholar]
- 37.Johnson K, Terkeltaub R. Inorganic pyrophosphate (PPI) in pathologic calcification of articular cartilage. Front Biosci. 2005;10:988–997. doi: 10.2741/1593. [DOI] [PubMed] [Google Scholar]
- 38.Karsenty G. The genetic transformation of bone biology. Genes Dev. 1999;13:3037–3051. doi: 10.1101/gad.13.23.3037. [DOI] [PubMed] [Google Scholar]
- 39.Olsen BR, Reginato AM, Wang W. Bone development. Annu Rev Cell Dev Biol. 2000;16:191–220. doi: 10.1146/annurev.cellbio.16.1.191. [DOI] [PubMed] [Google Scholar]
- 40.Fazzalari NL, Moore AJ, Byers S, Byard RW. Quantitative analysis of trabecular morphogenesis in the human costochondral junction during the postnatal period in normal subjects. Anat Rec. 1997;248:1–12. doi: 10.1002/(SICI)1097-0185(199705)248:1<1::AID-AR1>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 41.Shapiro F, Holtrop ME, Glimcher MJ. Organization and cellular biology of the perichondrial ossification groove of ranvier: A morphological study in rabbits. J Bone Joint Surg Am. 1977;59:703–723. [PubMed] [Google Scholar]
- 42.Schollmeier G, Uhthoff HK, Lewandrowski KU, Fukuhara K. Role of bone bark during growth in width of tubular bones. A study in human fetuses. Clin Orthop Relat Res. 1999:291–299. [PubMed] [Google Scholar]