Abstract
Background and purpose:
Inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) have been suggested as key components in various inflammatory diseases. Here we examined the effects of new quinolinedione derivatives, 6-(4-fluorophenyl)-amino-5,8-quinolinedione (OQ1) and 6-(2,3,4-trifluorophenyl)-amino-5,8-quinolinedione (OQ21) on activity and expression of iNOS and COX-2 to explore their anti-inflammatory properties.
Experimental approach:
The effects of OQ1 and OQ21 were assessed on lipopolysaccharide (LPS)-induced iNOS and COX-2 in murine macrophage cell line (RAW264.7), along with isolated enzyme assays to measure enzyme inhibition. Nuclear factor-κB (NFκB) activation pathways were investigated to elucidate mechanisms underlying OQ-mediated suppression of the expression of iNOS and COX-2. In vivo anti-inflammatory activities of OQ compounds were evaluated in mouse ear oedema, induced by topical 12-O-tetradecanoylphorbol-13-acetate (TPA).
Key results:
LPS-induced NO production in RAW264.7 cells was inhibited by OQ1 and OQ21 through the attenuation of iNOS expression as well as iNOS activity. Down-regulation of iNOS followed blocking of NFκB activation, as assessed by inhibitory κB degradation and electrophoretic mobility shift assay for NFκB. Synthesis and accumulation of prostaglandin E2 were also suppressed by OQ1 and OQ21. LPS-induced COX-2 expression and cellular COX-2 activities were attenuated by OQ1 and OQ21. Consistent with these results, OQ1 showed potent anti-inflammatory effects in mouse ear oedema induced by TPA.
Conclusions and implications:
The novel quinolinedione derivatives, OQ1 and OQ21, showed potent anti-inflammatory activity through dual inhibitory effects on iNOS and COX-2, suggesting that OQ derivatives might provide a new therapeutic modality for chronic inflammatory diseases, refractory to conventional drug therapies.
Keywords: iNOS, cyclooxygenase-2, anti-inflammatory agent, inflammation, TPA-induced mouse ear oedema, quinolinedione
Introduction
The enzymes, inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2), whose expression can be induced by cytokines or endotoxin (lipopolysaccharide, LPS), share many cellular and molecular features in common and are deeply involved in the inflammatory processes and inflammation-mediated tissue damage, due to the excessive production of NO and pro-inflammatory prostaglandins (Szabo and Thiemermann, 1994; Vane et al., 1994; Zamora et al., 2000). Concomitantly increased expression of iNOS and COX-2 is frequently observed in a wide range of inflammatory diseases, including endotoxin-mediated septic shock, rheumatoid arthritis and inflammatory vascular diseases, suggesting a considerable degree of cross talk and concerted action between these enzymes in the progression of inflammation (Salvemini et al., 1993; Goodwin et al., 1999; Uno et al., 2004; Mollace et al., 2005). In this context, a compound that could inhibit both iNOS and COX-2 would have great potential for wide and versatile application to many inflammatory or chronic immune disorders (Salvemini et al., 1995; Herencia et al., 1999; Chen et al., 2001).
Various molecular targets have been explored for the pharmacological manipulation of iNOS or COX-2. The promoter sequences of iNOS and COX-2 contain a binding site for the universal transcription factor, nuclear factor-κB (NFκB), which can be activated by cytokines, mitogenic mediators or endotoxins (Wu et al., 2005; Chen et al., 2006). Nuclear translocation of NFκB and subsequent transcriptional changes are accomplished by the activation of the inhibitory κB kinase–β complex through the degradation of inhibitory κBα (IκBα) and liberation of NFκB (Griscavage et al., 1996). As the modulation of NFκB pathways could affect a variety of inflammatory signalling pathways, it has been an attractive therapeutic target for anti-inflammatory drugs, especially in terms of the control of iNOS and COX-2 expression (Huang et al., 2001; Ukil et al., 2006). Direct inhibition of enzymic activities of iNOS and COX-2 has also been actively pursued as another efficient pharmacological approach, as exemplified by the active development of COX-2 inhibitors such as celecoxib (Deeks et al., 2002) and iNOS inhibitors (Alderton et al., 2005; Strub et al., 2006) for a wide range of chronic inflammatory diseases.
Previously, novel quinolinedione derivatives, 6-(4-fluorophenyl)-amino-5,8-quinolinedione (OQ1) and 6-(2,3,4-trifluorophenyl)-amino-5,8-quinolinedione (OQ21) were reported to inhibit L-arginine-induced endothelium-independent relaxation in aortic rings isolated from LPS-treated rats possibly through the inhibition of LPS-induced production of NO, a potent vasorelaxant (Malta et al., 1988; Lee et al., 2000). These studies suggested the therapeutic potential of the compounds to suppress LPS-mediated inflammatory responses. In the present study, we present the inhibitory effects of OQ1 and OQ21 on two inflammatory enzymes, iNOS and COX-2, in a LPS-stimulated macrophage cell line (RAW264.7). OQ1 and OQ21 suppressed the expression of both iNOS and COX-2 induced by LPS. This suppression was well-correlated with the inhibitory effect on NFκB activation and the enzymic activity of iNOS was also attenuated by these compounds. Of note, the in vivo study using 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced ear oedema in mice showed alleviation of inflammatory symptoms by treatment with OQ1 and OQ21, suggesting their potential as novel anti-inflammatory agents.
Methods
Cell culture
The mouse macrophage cell line, RAW264.7 was purchased from The American Type Culture Collection (Manassas, VA) and maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% heat-inactivated foetal bovine serum and 1% penicillin/streptomycin at 37°C under 5% CO2 atmosphere. Assays were conducted with cells harvested at 80–90% confluence.
Measurement of NO and prostaglandin E2 accumulation
For NO measurement, RAW264.7 cells were cultured overnight at 1 × 106 cells·mL−1 and then treated with LPS (0.1 µg·mL−1) alone or LPS with various concentrations of OQ1 or OQ21, which were determined to be free of cytotoxicity for 24 h. The cell supernatants were collected at the end of incubation for nitrite assay according to a previous report (Eigler et al., 1995). Briefly, equal volume of Griess reagent was mixed with cell supernatant (100 µL), and optical density at 540 nm was measured. The concentration of nitrite was calculated from the standard curve obtained with known concentrations of sodium nitrite dissolved in DMEM. For prostaglandin PGE2 measurement, cells were seeded at 5 × 105 cells·mL−1 and then treated with LPS (0.1 µg·mL−1) for 24 h. The levels of PGE2 were determined from supernatants using PGE2 EIA kit according to the manufacturer's instruction (detection range, 19.6–1250 pg·mL−1, R&D Systems, Minneapolis, MN).
Assay of iNOS and COX-2 activities in RAW264.7 cells
The iNOS and COX-2 activities were measured according to the previous report (Chen et al., 2001) with minor modifications. To induce iNOS, RAW264.7 cells were stimulated with LPS for 16 h and washed twice with fresh media to remove LPS. Cells were further incubated with OQ1 and OQ21 for 8 h and thereafter, the levels of nitrites were measured using Griess reagent to determine iNOS activity. For measurement of COX-2 enzymic activity, cells were treated with LPS for 8 h, and washed twice with fresh media. Cells were further treated with OQ1 or OQ21 for 30 min and washed twice with new media. PG synthesis was initiated with the addition of arachidonic acid (30 µmol·L−1) and the levels of PGE2 were determined using PGE2 EIA kit.
iNOS enzyme assay using isolated murine macrophage iNOS
The activity of iNOS was measured by the reaction of NO with oxyhaemoglobin (oxyHb) to form methaemoglobin (metHb) as described before (Alderton et al., 2005). To prepare oxyHb, human haemoglobin was dissolved in water at concentrations of 1 mmol·L−1 and oxygenated for 10 min by bubbling with O2 gas. Human haemoglobin was then reduced with sodium dithionite (10 mmol·L−1) and reoxygenated with O2 gas for additional 15 min. Sodium dithionite was removed on a Sephadex G25 column (GE Healthcare, Piscataway, NJ) to get pure oxyHb and formation of metHb was initiated by the addition of NADPH (100 µmol·L−1) to reaction buffer [50 mmol·L−1 HEPES containing 1 mmol·L−1 arginine, 1 mmol·L−1 magnesium acetate, 5 µmol·L−1 oxyHb, 12 µmol·L−1 tetrahydrobiopterin (BH4), 170 µmol·L−1 dithiothreitol (DTT) and 1 U·mL−1 purified murine macrophage iNOS, pH 7.4]. The conversion of oxyHb to metHb was monitored with the increase in absorbance at 401 nm, using an extinction coefficient of 60 000 M−1·cm−1. Effect of OQs on iNOS activity was analysed after treatment with OQs for 5 min.
COX-1/-2 enzyme assay
Direct inhibitory activities against COX enzyme were measured using commercially available colorimetric COX (ovine) inhibitor screening kit (Cayman Chemical, Ann Arbor, MI) according to the manufacturer's protocol. Briefly, after COX-1 or COX-2 enzyme was incubated with OQ1 and OQ21 for 30 min, reaction was initiated with addition of arachidonic acid (100 µmol·L−1).
Western blot analysis
RAW264.7 cells were cultured (4 × 106 cells·mL−1) in 60 mm tissue culture dish for 24 h, followed by treatment with LPS alone or LPS plus various concentrations of OQ1 or OQ21. At the end of incubation, cells were washed with ice-cold phosphate-buffered saline (PBS) and solubilized in cold lysis buffer [50 mmol·L−1 Tris-HCl (pH 7.4), 1 mmol·L−1 diethyldithiocarbamic acid, 10 mmol·L−1 EDTA, 1 mmol·L−1 phenylmethylsulphonylfluoride (PMSF), 1% Tween20, 1% Triton X-100, 10 µmol·L−1 leupeptin] for 20 min on ice. Cell lysates were harvested with a cell scraper and centrifuged at 10 000× g for 20 min at 4°C. For the Western blot analysis of phospho-p38 and phospho-Erk, cells were lysed with RIPA buffer (1% Nonidet P-40, 0.5% sodium deoxycholate and 0.1% sodium dodecyl sulphate in PBS, pH 7.4) containing 0.1 mmol·L−1 Na3VO4 and protease inhibitors (0.5 mg·mL−1 aprotinin, 0.5 mg·mL−1 E-64, 0.5 mg·mL−1 pepstatin, 0.5 mg·mL−1 bestatin, 10 mg·mL−1 chymostatin and 0.1 mg·mL−1 leupeptin) to facilitate the detection of the phosphorylated proteins. Protein concentration in supernatant was measured using Bio-Rad protein assay reagent (Bio-Rad, Hercules, CA). A quantity of 20 µg proteins was separated by electrophoresis using sodium dodecyl sulphate polyacrylamide gel and transferred to polyvinylidene fluoride membrane. Each protein was identified with polyclonal antibodies against iNOS, COX-2, IκBα, Erk, pErk (dilution, 1:1000 for iNOS, COX-2, IκBα, 1:500 for Erk, pErk; Santa Cruz Biotech, Santa Cruz, CA), tubulin (dilution, 1:5000; Calbiochem, Germany), p38, pp38 (dilution, 1:1000; Upstate, Charlottesville, VA) or secondary antibodies conjugated with horseradish peroxidase (dilution, 1:2000; Zymed Lab, San Francisco, CA) using ECL kit (Amersham Biosciences, Inc.).
Preparation of nuclear extracts
After OQ1 or OQ21 was treated with LPS for 3 h, nuclear extracts from 1 × 106 cells were prepared according to the previously reported method (Schreiber et al., 1990). Briefly, cells were washed with ice-cold PBS, and allowed to swell after the addition of 100 µL hypotonic buffer [10 mmol·L−1 HEPES (pH 7.9), 10 mmol·L−1 KCl, 0.1 mmol·L−1 EDTA, 0.5% Nonidet P-40, 1 mmol·L−1 DTT and 0.5 mmol·L−1 PMSF] for 10 min. The lysates were centrifuged at 7200× g for 5 min at 4°C. Pellets were re-suspended in 50 µL of hypertonic buffer [20 mmol·L−1 HEPES (pH 7.9), 400 mmol·L−1 NaCl, 1 mmol·L−1 EDTA, 10 mmol·L−1 DTT and 1 mmol·L−1 PMSF] and then incubated further for 30 min in ice. The resultant supernatants from centrifugation at 15 000× g for 10 min were obtained as nuclear fractions. Concentration of protein in supernatant was measured using Bio-Rad protein assay reagent.
Electrophoretic mobility shift assay
Double-stranded oligonucleotides containing NFκB binding site (5′-AGTTGAGGGGACTTTCCCAGGC-3′) were end-radiolabelled using T4 polynucleotide kinase and [32P]-ATP. Labelled oligonucleotides and 5 µg of nuclear extracts were incubated with 2 µL of 5× binding buffer (20% glycerol, 5 mmol·L−1 MgCl2, 250 mmol·L−1 NaCl, 2.5 mmol·L−1 EDTA, 2.5 mmol·L−1 DTT, 0.25 mg·mL−1 poly dI-dC and 50 mmol·L−1 Tris-Cl, pH 7.5) in a total volume of 15 µL of reaction mixture at 25°C for 30 min. For competition experiments, unlabelled double-stranded oligonucleotides were pre-incubated in 20-fold excess with the protein for 20 min before labelled oligonucleotides were added. All samples were analysed by electrophoresis on 4% native polyacrylamide gels for 1.5 h at 140 V. The gel was removed, fixed and dried, followed by autoradiography.
Transfection and luciferase reporter gene assay
RAW264.7 cells were transfected with a murine COX-2 promoter (−3.2 kb) luciferase plasmid using Superfect transfection reagent (Qiagen, Valencia, CA) according to the manufacturer's instructions as described previously (Lee et al., 2004). HSP70-β-galactosidase plasmid was co-transfected as an internal control. The total amount of transfected plasmids was equalized by supplementing with the corresponding empty vector. Luciferase and β-galactosidase enzyme activities were determined using the Luciferase Assay System and β-galactosidase Enzyme System (Promega, Madison, WI) according to the manufacturer's instructions. Luciferase activity was normalized against β-galactosidase activity.
TPA-induced mouse ear oedema model
Protocols for animal experiments were approved by the Ethics Committee of Animal Service Center at Seoul National University. The study using TPA-induced ear oedema model was conducted according to the previous report (De Young et al., 1989) with a minor modification. Male ICR mice (SamTaco, Korea) were randomly assigned to four groups (10 mice in each group). TPA, dissolved in dimethylsulphoxide: acetone (1:9, 125 µg·mL−1) was applied to inner and outer sides of the right ear of each mouse (2.5 µg per ear). After 30 min and 6 h from the time of TPA application, indomethacin, OQ1, OQ21 dissolved in acetone (0.1 mg in 20 µL, 0.5%, solubility limit of OQ21) or vehicle was painted on the right ear. After 24 h from TPA stimulus, 6 mm ear pad biopsy was collected from the right ear of each mouse with a coring tool (Stipel Co., Korea) and weighed for the determination of severity of oedema. Biopsies of the left ears of TPA group were collected for normal control. After weighing, biopsies were fixed in 10% neutral buffered formalin and examined histologically after staining with haematoxylin and eosin.
Statistical analysis
Western blot data or histological data are presented with representative figures from more than three independent experiments. Other data were shown as mean ± SEM from more than three experiments. Difference between each groups was examined using one-way anova followed by Duncan's multiple range test (significant when P < 0.05). Histological scoring data were analysed by non-parametric Mann–Whitney U test to determine whether the score difference between two groups was statistically significant (P < 0.05).
Reagents
DMEM, penicillin/streptomycin and foetal bovine serum were obtained from Gibco BRL (Grand Island, NY). PGE2 enzyme immunoassay kit was purchased from R&D Systems (Minneapolis, MN). Anti-rabbit IgG conjugated with horseradish peroxidase was purchased from Amersham (Arlington Heights, IL). Purified murine macrophage iNOS and ovine COX-1/-2 assay kit were bought from Cayman Chemical (Ann Arbor, MI). LPS, Griess reagent, arachidonic acid, dimethylsulphoxide, indomethacin, TPA, human haemoglobin, sodium dithionite, NADPH, HEPES, arginine, magnesium acetate, tetrahydrobiopterin (BH4), DTT, salts for buffer preparation and other reagents were all from Sigma Chemical Co. (St. Louis, MO). OQ1 and OQ21 were generous gifts from Professor Chung-Kyu Ryu at Ewha Womans University, Korea.
Results
Inhibition of LPS-induced nitric oxide production by OQ1 and OQ21 mediated by suppression of the enzyme activity and the expression level of iNOS
To investigate if OQ1 and OQ21 have anti-inflammatory activities, LPS-induced nitric oxide (NO) production was determined in the presence of OQ1 or OQ21 (1, 5, 10 and 25 µmol·L−1) in RAW264.7 mouse macrophage cells. LPS-induced nitrite generation, an indicator for NO production, was significantly attenuated by OQ1 and OQ21 treatment, from as low as 5 µmol·L−1 (Figure 1A), suggesting potent anti-inflammatory activities of these compounds.
Figure 1.
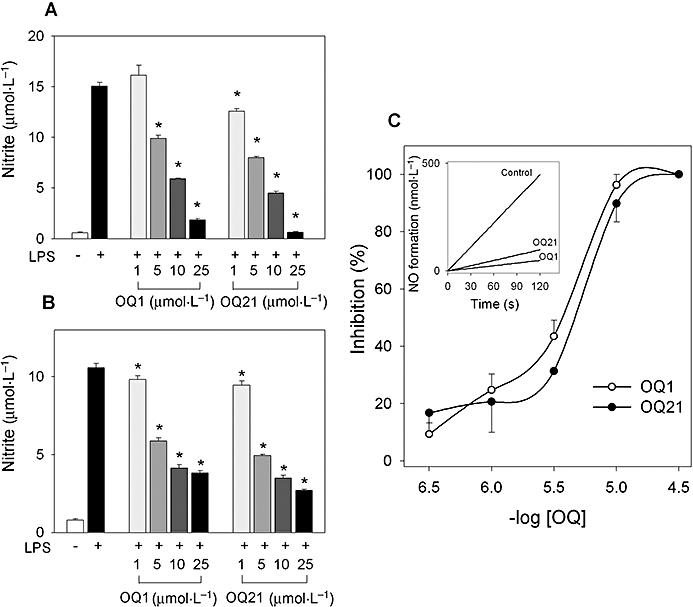
The suppressive effects of OQ1 and OQ21 on production of nitric oxide (NO) and enzyme activity of iNOS. A. RAW264.7 cells were incubated with LPS (0.1 µg·mL−1) in the presence of OQ1 or OQ21 (1, 5, 10 and 25 µmol·L−1) for 24 h. Accumulated nitrite was measured using Griess reagent. B. RAW264.7 cells were pre-incubated with LPS (0.1 µg·mL−1) for 16 h to induce iNOS. After cells were washed with fresh media to remove LPS, cells were further treated with OQ1 or OQ21 for 8 h and nitrite generation was determined. C. Purified murine iNOS was incubated with OQ1 or OQ21 for 5 min and iNOS activity was measured by NADPH-initiated conversion of oxyHb to metHb. Values are means ± SEM (n = 4–5). * represents a significant difference from LPS-treated control by one-way anova followed by Duncan's multiple range test (P < 0.05). OQ1, 6-(4-fluorophenyl)-amino-5,8-quinolinedione; OQ21, 6-(2,3,4-trifluorophenyl)-amino-5,8-quinolinedione; iNOS, inducible nitric oxide synthase; LPS, lipopolysaccharide.
The inhibition of NO production by OQ compounds can result from the suppression of the enzymic activity and/or the expression level of iNOS. First, we investigated whether enzymic activity of iNOS was affected by OQ1 and OQ21. To determine if iNOS activity in cellular system could be affected by OQs, RAW264.7 cells were stimulated with LPS to induce iNOS. After LPS was removed, cells were further treated with OQ1 and OQ21 and the production of NO was determined as the level of nitrite. OQ1 and OQ21 reduced the generation of nitrite in LPS-stimulated cells in a concentration-dependent manner (Figure 1B), suggesting that these compounds may inhibit the enzymic activity of iNOS in cellular systems. These results were further confirmed with assays for in vitro iNOS enzyme activity. OQ1 and OQ21 suppressed the activity of the purified iNOS dose-dependently with IC50 values of 3.2 ± 1.2 µmol·L−1 and 5.3 ± 2.8 µmol·L−1 respectively (Figure 1C). These inhibitory effects were not due to non-specific cytotoxicities of OQ1 or OQ21, as determined by cytotoxicity measurement [3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl tetrazolium bromide assay, data not shown].
Next, we investigated whether inhibition of NO production by OQs stemmed from the decreased expression of iNOS. As shown in Figure 2A, LPS-induced iNOS expression was decreased by OQ1 and OQ21 treatment in a concentration-dependent manner and OQ1 was the more potent inhibitor. The NFκB activation pathway is importantly involved in the regulation of iNOS expression in macrophage cell (Huang et al., 2001). Therefore, we determined if the suppression of iNOS expression by OQ1 and OQ21 was mediated by the modulation of NFκB activation. As shown in Figure 2B,C, OQ1 and OQ21 inhibited NFκB activation induced by LPS, as determined by both IκBα degradation assay and electrophoretic mobility shift assay.
Figure 2.
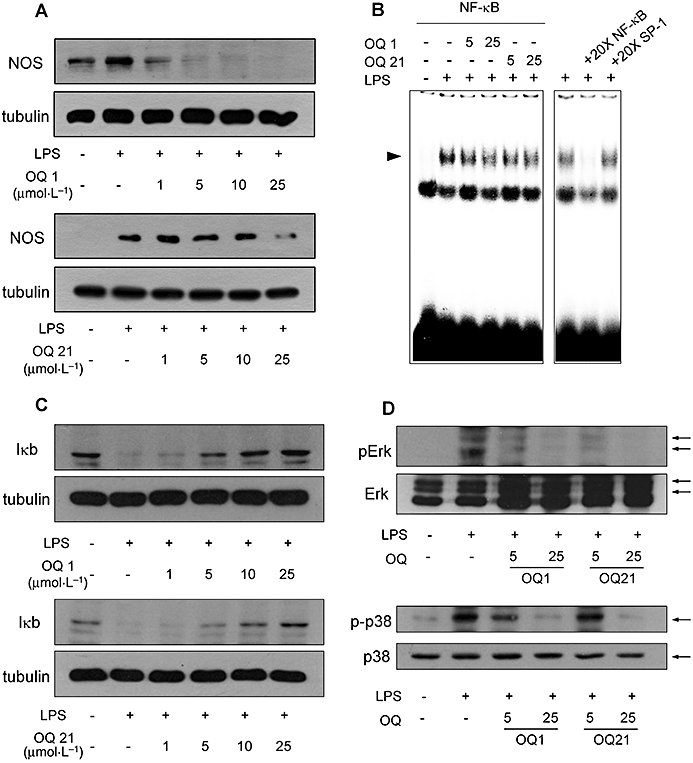
Suppression of iNOS expression and upstream signalling pathways by OQ1 and OQ21 in LPS-stimulated macrophages. A. RAW264.7 cells were incubated with LPS (0.1 µg·mL−1) and various concentrations of OQ1 or OQ21 for 8 h. iNOS expression was determined by immunoblot assay with α-tubulin as a loading control. B. LPS-induced activation of NFκB was determined by electrophoretic mobility shift assay from nuclear extracts prepared from the cells stimulated with LPS (0.1 µg·mL−1) in the presence of OQ1 or OQ21 (25 µmol·L−1) for 3 h. Competition experiments were performed by adding 20-fold excess amount of unlabelled oligonucleotides containing the consensus binding sequence for NFκB or SP-1. C and D. RAW264.7 cells incubated with LPS (0.1 µg·mL−1) and various concentrations of OQ1 or OQ21 for 30 min. Cell lysates were analysed for IκBα, phospho-Erk, and phospho-p38 by immunoblot assay. Representative results are shown from more than three separate experiments for each assay. OQ1, 6-(4-fluorophenyl)-amino-5,8-quinolinedione; OQ21, 6-(2,3,4-trifluorophenyl)-amino-5,8-quinolinedione; iNOS, inducible nitric oxide synthase; LPS, lipopolysaccharide; NFκB, nuclear factor-κB.
It has been reported that mitogen-activated protein kinases, Erk and p38 play a role in the expression of iNOS in LPS-stimulated macrophages (Ajizian et al., 1999). In our experiments, OQ1 and OQ21 suppressed the phosphorylation of Erk and p38 induced by LPS (Figure 2D), showing that OQ1 and OQ21 regulated upstream signalling pathways to attenuate LPS-induced iNOS expression in macrophages. Collectively, our results demonstrated that OQ1 and OQ21 were able to suppress both enzyme activity and the expression level of iNOS in LPS-stimulated macrophages.
Inhibition of LPS-induced PGE2production by OQ1 and OQ21 in macrophage cells
COX-2 is another important pro-inflammatory enzyme, which can be induced in LPS-stimulated macrophages (Lee et al., 1992), as shown by the extensive production of inflammatory PGs. To determine if OQ1 and OQ21 could reduce PG production induced by LPS, RAW264.7 cells were stimulated with LPS in the presence of OQ1 or OQ21. The levels of PGE2, a representative inflammatory PG generated by the COX-2 pathway, were significantly decreased by OQ1 and OQ21 treatment, in a concentration-dependent manner from as low as 1 µmol·L−1 (Figure 3A).
Figure 3.
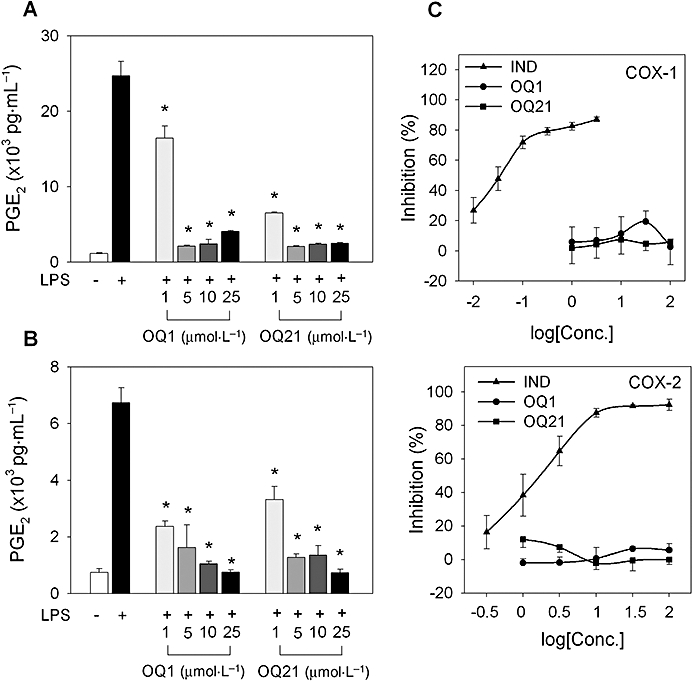
The effects of OQ1 and OQ21 on LPS-induced PGE2 production and enzyme activity of COX-2. A. RAW264.7 cells were incubated with LPS (0.1 µg·mL−1) and various concentrations of OQ1 or OQ21 for 8 h. The levels of accumulated PGE2 were determined in the medium by PGE2 EIA. B. RAW264.7 cells were stimulated with LPS for 8 h to induce COX-2 and washed with fresh media to remove LPS. Cells were further treated with OQ1 or OQ21 for 30 min followed by the addition of arachidonic acid (30 µmol·L−1) as a substrate for COX-2. After 15 min, the levels of PGE2 in media were determined using PGE2 EIA. C. After purified COX-1/-2 enzymes were incubated with OQ1, OQ21 or indomethacin (IND) for 30 min, COX activities were measured by colorimetric assessment. Values are means ± SEM (n = 4–5). * represents a significant difference from LPS-treated control by one-way anova followed by Duncan's multiple range test (P < 0.05). OQ1, 6-(4-fluorophenyl)-amino-5,8-quinolinedione; OQ21, 6-(2,3,4-trifluorophenyl)-amino-5,8-quinolinedione; LPS, lipopolysaccharide; PGE2, prostaglandin E2; COX, cyclooxygenase.
To investigate if the suppressive effects of OQ compounds on PGE2 production resulted from the modification of enzyme activity of COX-2 by OQs, we determined whether cellular activities of COX-2 were affected. After COX-2 was pre-induced with LPS challenge in RAW264.7 cells, cells were incubated with OQ1 or OQ21 for 30 min, and PGE2 production was primed with exogenous arachidonic acid. Under these conditions, PGE2 production in LPS-stimulated cells was inhibited by OQ1 or OQ21, suggesting the suppressive effects of these compounds on enzymatic activity of COX-2 in cellular system (Figure 3B). In contrast to the results from whole cells, in vitro enzyme assays using purified COX-2 showed that COX-2 activities were not affected by OQ1 or OQ21 (Figure 3C). These results suggest that the inhibitory effects of OQ compounds on cellular COX-2 activity may be mediated by the action of OQ metabolites or the activation of secondary pathways by OQs, but not by the direct interaction between OQs and COX-2 enzyme.
To investigate if the decrease in PGE2 production was due to the lowered expression of COX-2, RAW264.7 cells were stimulated with LPS in the presence of OQ1 or OQ21. LPS-induced COX-2 expression was attenuated by OQ1 or OQ21 as determined by immunoblot assay and COX-2 promoter reporter assay (Figure 4A,B). These results showed that OQ1 and OQ21 prevented the increased production of PGE2 possibly due to the suppression of both enzyme activity and protein expression of COX-2.
Figure 4.
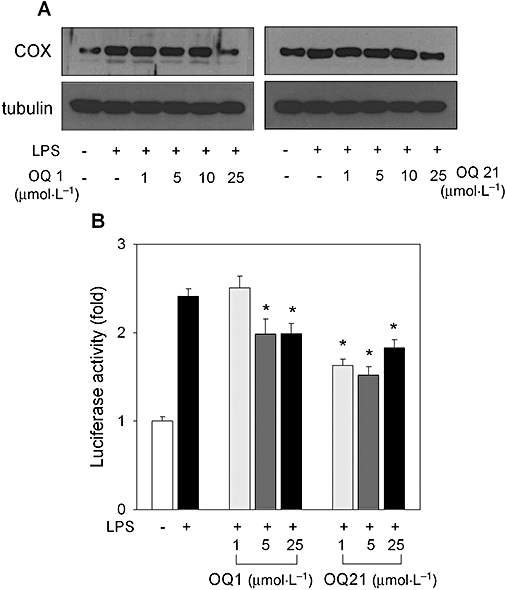
Effects of OQ1 or OQ21 on COX-2 expression in LPS-stimulated macrophages. A. RAW264.7 cells were incubated with LPS (0.1 µg·mL−1) and various concentrations of OQ1 or OQ21 for 8 h. COX-2 expression was determined by immunoblot assay with α-tubulin as a loading control. Representative results from more than three separate experiments are shown. B. RAW264.7 cells were transfected with murine COX-2 promoter (−3.2 kb) luciferase reporter plasmid and β-galactosidase expression plasmid. Cells were further treated with LPS (0.1 µg·mL−1) and various concentrations of OQ1 or OQ21 for 8 h. Cell lysates were analysed for luciferase and β-galactosidase enzyme activities. Fold induction was calculated after normalization with β-galactosidase activity. Values are mean ± SEM (n = 4). * represents a significant difference from LPS-treated group by one-way anova followed by Duncan's multiple range test (P < 0.05). OQ1, 6-(4-fluorophenyl)-amino-5,8-quinolinedione; OQ21, 6-(2,3,4-trifluorophenyl)-amino-5,8-quinolinedione; LPS, lipopolysaccharide; COX-2, cyclooxygenase-2.
In vivo efficacy of OQ1 in TPA-induced mouse ear oedema
To investigate if the suppressive effects of OQ compounds on iNOS and COX-2 could lead to anti-inflammatory activities in vivo, we used a topical inflammation model, TPA-induced mouse ear oedema. Topically applied TPA induced oedema in mouse ear and increased weights of ear biopsies, while the application of OQ1 reversed the increased weight induced by TPA (Figure 5A). Similarly, OQ21 treatment attenuated the increase of ear weight, although it failed to show statistically significant results.
Figure 5.
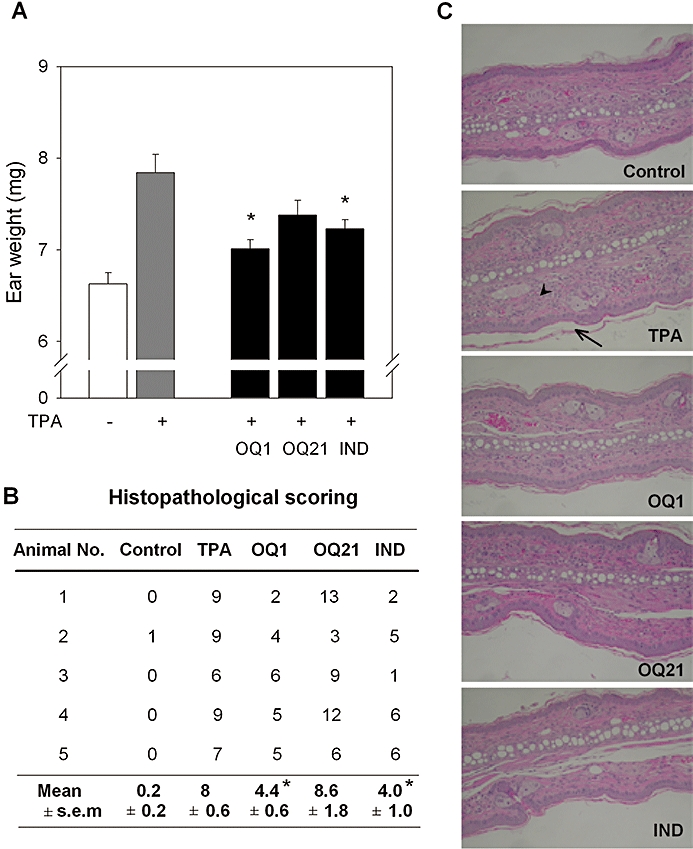
Anti-inflammatory activity of OQ1 in TPA-induced ear oedema mouse model. A. Ear oedema was induced with topical application of TPA (2.5 µg per ear). OQ1, OQ21 or indomethacin (IND; 0.1 mg per ear, 10 animals per group) was painted on the ear pad, 30 min and 6 h after TPA treatment. Twenty-four hours after TPA application, ear biopsies were collected and weighed to determine the severity of oedema formation. Values are means ± SEM (n = 10). * represents a significant difference from TPA-treated group by one-way anova followed by Duncan's multiple range test (P < 0.05). B. Five ear biopsies were randomly selected per group and processed (haematoxylin and eosin staining) for histological examinations. Tailless arrowhead represents inflammatory cells and tailed arrow, hyperkeratosis. Representative microscopic photographs are presented. C. Histopathological changes were scored for dermis and epidermis. Epidermal changes were evaluated with the scores for thickness (0–5), stratum granulosum (0–3), hyperkeratosis (0–5), spongiosis (0–4) and intracellular oedema (0–1). Dermal changes were scored for infiltration (0–6). Total scores for the respective ear are presented. * represents a significant difference from TPA-treated group by the Mann–Whitney U tests (P < 0.05). OQ1, 6-(4-fluorophenyl)-amino-5,8-quinolinedione; OQ21, 6-(2,3,4-trifluorophenyl)-amino-5,8-quinolinedione; TPA, 12-O-tetradecanoylphorbol-13-acetate.
The histopathological assessment of the skin inflammation also confirmed the potent anti-inflammatory efficacy of OQ1, where the infiltrations of inflammatory cells, thickening of stratum corneum and epidermal hyperkeratosis, important histological features of topical inflamation were all reduced (Figure 5B,C). Notably, its potency was comparable with indomethacin, a known COX-1/-2 inhibitor, suggesting possible synergistic effects of iNOS and COX-2 inhibition by OQ1 on the control of topical inflammatory conditions.
Discussion
In the present study, we demonstrated anti-inflammatory activities of two quinolinedione derivatives, OQ1 and OQ21, in both in vitro and in vivo experimental systems, using LPS-stimulated RAW264.7 macrophages and a mouse model of topical inflammation respectively. Dual inhibitory activities against iNOS and COX-2 as shown in in vitro assays appear to confer on OQ1 a potent in vivo efficacy in mouse, TPA-induced, ear oedema, comparable with a potent and well-known COX inhibitor, indomethacin, suggesting its potential therapeutic usage as a novel topical anti-inflammatory drug.
Excessive production of NO and PGE2 plays a critical role in the aggravation of circulatory shock and chronic inflammatory diseases, such as septic shock, inflammatory hepatic dysfunctions (Wu et al., 1995; Liaudet et al., 1998), inflammatory lung disease and colitis (Dugo et al., 2004; Di Paola et al., 2005). As many of these conditions exhibit rapid onset and development, often resulting in the failure of conventional anti-inflammatory therapies and extremely high mortality rates (Rivers et al., 2001), a simultaneous suppression of NO and PGE2production pathways, as shown by OQ1 and OQ21, may satisfy the so far unmet need for control of the rapid progression of the inflammatory process.
It is interesting that a single compound could suppress the protein synthesis and enzymic activity simultaneously, whereas most of the known iNOS inhibitors were targeted on either enzymic activity or protein expression through transcriptional modulation (Strub et al., 2006). Quinolinediones are known to have direct inhibitory efficacy against neuronal NOS as they could bind to the P450 reductase domain of NOS and shunt electrons from NADPH (Kumagai et al., 1998), suggesting a direct inhibition of iNOS enzyme activity by OQ1 and OQ21. Similar profiles of enzyme inhibition could be observed with the COX-2 inhibitor, celecoxib in TNFα-induced COX-2 in non-small cell lung carcinoma (Shishodia et al., 2004), where celecoxib suppressed the expression of COX-2 through the inhibition of inhibitory κB kinase activation, as well as COX-2 activity. The suppression of iNOS expression by OQ1 and OQ21 was also well-correlated with the inhibition of NFκB pathways. Other data, such as IκB degradation and decreased NFκB nuclear translocation, along with the suppression of mitogen-activated protein kinase pathways, suggest an overall suppression of NFκB pathways.
De novo synthesis and cellular accumulation of PGE2 in LPS-stimulated macrophages were suppressed by OQ1 and OQ21 in a concentration-dependent manner (Figure 3A,B). While COX-2 activity was decreased by OQ1 and OQ21 in cellular systems, the activity of purified COX-1/-2 enzyme was not affected by OQ compounds in in vitro assay system. It was quite unexpected as the inhibitory efficacy on COX-2 activities could be also confirmed in another cell-based assay system, mouse primary peritoneal macrophage COX-1/COX-2 assays, where OQ1 and OQ21 showed selectivity for COX-2, from 10- to 3.6-fold (COX-1/COX-2 IC50 values, >25 µmol·L−1/2.5 µmol·L−1 and >25 µmol·L−1/6.9 µmol·L−1 for OQ1 and OQ21 respectively) (Chung et al., unpubl. data). The discrepancy between cellular and isolated enzyme assays could be explained in part by the results from a previous report that decreased NO following iNOS inhibition attenuated PG formation, because of the absence of the enhancement of PG synthesis by NO (Mollace et al., 2005). Although a detailed biochemical and analytical study is required, we could also speculate on the possible generation of active metabolites of OQ1 and OQ21 by intracellular metabolism, as has been suggested in the case of menadione (Barchowsky et al., 1989).
Consistent with the in vitro anti-inflammatory effects, OQ1 potently attenuated TPA-induced mouse ear oedema, while OQ21 showed weaker activity. The lower solubility and poorer skin penetration due to two more fluoro atoms in the phenyl ring of OQ21 might explain the lower in vivo activity when compared with OQ1, although further study is necessary to confirm it. Although statistical significance was not achieved, epidermal hyperkeratosis, a typical feature of local inflammatory reaction in skin stratum corneum, was slightly decreased by OQ1 treatment (histopathological scorings, 1.2 ± 0.4 and 0.4 ± 0.6 for TPA and OQ1 group respectively, P = 0.056, by Mann–Whitney U test), in which the more potent COX inhibitor, indomethacin, showed little effect (0.8 ± 0.8, P = 0.41). Hyperkeratosis and keratinocyte proliferation are common features in various chronic inflammatory diseases of skin, such as psoriasis or atopic dermatitis (Hansson et al., 2002). This additional benefit, the alleviation of hyperkeratosis, might come from the dual inhibition of iNOS and COX-2 or NFκB down-regulation by OQ1, which cannot be provided by a pure COX inhibitor like indomethacin.
In conclusion, our study shows that OQ derivatives with dual inhibitory effects against iNOS and COX-2 could be powerful therapeutic options for acute and chronic inflammatory diseases, which have been refractory to conventional drug therapies. It also supports the idea that combination therapy with iNOS and COX-2 inhibitors could provide a therapeutic synergy in the treatment of topical inflammatory diseases.
Acknowledgments
This work was supported by the SRC/ERC Programme of MOST/KOSEF (R11-2007-107-01002-0).
Glossary
Abbreviations:
- COX-2
Cyclooxygenase-2
- DMEM
Dulbecco's modified Eagle's medium
- EMSA
Electrophoretic mobility shift assay
- HEPES
N-2-hydroxyethylpiperazine-N'-2-ethanolsulfonic acid
- IκB
inhibitory κB
- IKK
inhibitory κB kinase
- LPS
lipopolysaccharide
- MAPK
mitogen-activated protein kinase
- MTT
3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl tetrazolium bromide
- OQ1
6-(4-fluorophenyl)-amino-5,8-quinolinedione
- OQ21
6-(2,3,4-trifluorophenyl)-amino-5,8-quinolinedione
- PBS
Phosphate-buffered saline
- PVDF
Polyvinylidene fluoride
- SDS
sodium dodecyl sulphate
- TPA
12-O-tetradecanoylphorbol-13-acetate
Conflicts of interest
The authors state no conflicts of interest.
References
- Ajizian SJ, English BK, Meals EA. Specific inhibitors of p38 and extracellular signal-regulated kinase mitogen-activated protein kinase pathways block inducible nitric oxide synthase and tumor necrosis factor accumulation in murine macrophages stimulated with lipopolysaccharide and interferon-gamma. J Infect Dis. 1999;179:939–944. doi: 10.1086/314659. [DOI] [PubMed] [Google Scholar]
- Alderton WK, Angell AD, Craig C, Dawson J, Garvey E, Moncada S, et al. GW274150 and GW273629 are potent and highly selective inhibitors of inducible nitric oxide synthase in vitro and in vivo. Br J Pharmacol. 2005;145:301–312. doi: 10.1038/sj.bjp.0706168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barchowsky A, Tabrizi K, Kent RS, Whorton AR. Inhibition of prostaglandin synthesis after metabolism of menadione by cultured porcine endothelial cells. J Clin Invest. 1989;83:1153–1159. doi: 10.1172/JCI113995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CH, Sheu MT, Chen TF, Wang YC, Hou WC, Liu DZ, et al. Suppression of endotoxin-induced proinflammatory responses by citrus pectin through blocking LPS signaling pathways. Biochem Pharmacol. 2006;72:1001–1009. doi: 10.1016/j.bcp.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Chen YC, Shen SC, Lee WR, Hou WC, Yang LL, Lee TJ. Inhibition of nitric oxide synthase inhibitors and lipopolysaccharide induced inducible NOS and cyclooxygenase-2 gene expressions by rutin, quercetin, and quercetin pentaacetate in RAW 264.7 macrophages. J Cell Biochem. 2001;82:537–548. doi: 10.1002/jcb.1184. [DOI] [PubMed] [Google Scholar]
- De Young LM, Kheifets JB, Ballaron SJ, Young JM. Edema and cell infiltration in the phorbol ester-treated mouse ear are temporally separate and can be differentially modulated by pharmacologic agents. Agents Actions. 1989;26:335–341. doi: 10.1007/BF01967298. [DOI] [PubMed] [Google Scholar]
- Deeks JJ, Smith LA, Bradley MD. Efficacy, tolerability, and upper gastrointestinal safety of celecoxib for treatment of osteoarthritis and rheumatoid arthritis: systematic review of randomised controlled trials. Br Med J. 2002;325:619. doi: 10.1136/bmj.325.7365.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Paola R, Mazzon E, Patel NS, Genovese T, Muia C, Thiemermann C, et al. Beneficial effects of GW274150 treatment on the development of experimental colitis induced by dinitrobenzene sulfonic acid. Eur J Pharmacol. 2005;507:281–289. doi: 10.1016/j.ejphar.2004.11.041. [DOI] [PubMed] [Google Scholar]
- Dugo L, Marzocco S, Mazzon E, Di Paola R, Genovese T, Caputi AP, et al. Effects of GW274150, a novel and selective inhibitor of iNOS activity, in acute lung inflammation. Br J Pharmacol. 2004;141:979–987. doi: 10.1038/sj.bjp.0705683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eigler A, Moeller J, Endres S. Exogenous and endogenous nitric oxide attenuates tumor necrosis factor synthesis in the murine macrophage cell line RAW 264.7. J Immunol. 1995;154:4048–4054. [PubMed] [Google Scholar]
- Goodwin DC, Landino LM, Marnett LJ. Effects of nitric oxide and nitric oxide-derived species on prostaglandin endoperoxide synthase and prostaglandin biosynthesis. FASEB J. 1999;13:1121–1136. doi: 10.1096/fasebj.13.10.1121. [DOI] [PubMed] [Google Scholar]
- Griscavage JM, Wilk S, Ignarro LJ. Inhibitors of the proteasome pathway interfere with induction of nitric oxide synthase in macrophages by blocking activation of transcription factor NF-kappa B. Proc Natl Acad Sci USA. 1996;93:3308–3312. doi: 10.1073/pnas.93.8.3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson L, Backman A, Ny A, Edlund M, Ekholm E, Ekstrand Hammarstrom B, et al. Epidermal overexpression of stratum corneum chymotryptic enzyme in mice: a model for chronic itchy dermatitis. J Invest Dermatol. 2002;118:444–449. doi: 10.1046/j.0022-202x.2001.01684.x. [DOI] [PubMed] [Google Scholar]
- Herencia F, Ferrandiz ML, Ubeda A, Guillen I, Dominguez JN, Charris JE, et al. Novel anti-inflammatory chalcone derivatives inhibit the induction of nitric oxide synthase and cyclooxygenase-2 in mouse peritoneal macrophages. FEBS Lett. 1999;453:129–134. doi: 10.1016/s0014-5793(99)00707-3. [DOI] [PubMed] [Google Scholar]
- Huang YC, Guh JH, Cheng ZJ, Chang YL, Hwang TL, Liao CH, et al. Inhibition of the expression of inducible nitric oxide synthase and cyclooxygenase-2 in macrophages by 7HQ derivatives: involvement of IkappaB-alpha stabilization. Eur J Pharmacol. 2001;418:133–139. doi: 10.1016/s0014-2999(01)00922-0. [DOI] [PubMed] [Google Scholar]
- Kumagai Y, Midorikawa K, Nakai Y, Yoshikawa T, Kushida K, Homma-Takeda S, et al. Inhibition of nitric oxide formation and superoxide generation during reduction of LY83583 by neuronal nitric oxide synthase. Eur J Pharmacol. 1998;360:213–218. doi: 10.1016/s0014-2999(98)00666-9. [DOI] [PubMed] [Google Scholar]
- Lee JA, Jung SH, Bae MK, Ryu CK, Lee JY, Chung JH, et al. Pharmacological effects of novel quinone compounds, 6-(fluorinated-phenyl)amino-5,8-quinolinediones, on inhibition of drug-induced relaxation of rat aorta and their putative action mechanism. Gen Pharmacol. 2000;34:33–42. doi: 10.1016/s0306-3623(00)00044-6. [DOI] [PubMed] [Google Scholar]
- Lee JY, Zhao L, Youn HS, Weatherill AR, Tapping R, Feng L, et al. Saturated fatty acid activates but polyunsaturated fatty acid inhibits Toll-like receptor 2 dimerized with Toll-like receptor 6 or 1. J Biol Chem. 2004;279:16971–16979. doi: 10.1074/jbc.M312990200. [DOI] [PubMed] [Google Scholar]
- Lee SH, Soyoola E, Chanmugam P, Hart S, Sun W, Zhong H, et al. Selective expression of mitogen-inducible cyclooxygenase in macrophages stimulated with lipopolysaccharide. J Biol Chem. 1992;267:25934–25938. [PubMed] [Google Scholar]
- Liaudet L, Rosselet A, Schaller MD, Markert M, Perret C, Feihl F. Nonselective versus selective inhibition of inducible nitric oxide synthase in experimental endotoxic shock. J Infect Dis. 1998;177:127–132. doi: 10.1086/513813. [DOI] [PubMed] [Google Scholar]
- Malta E, Macdonald PS, Dusting GJ. Inhibition of vascular smooth muscle relaxation by LY83583. Naunyn Schmiedebergs Arch Pharmacol. 1988;337:459–464. doi: 10.1007/BF00169540. [DOI] [PubMed] [Google Scholar]
- Mollace V, Muscoli C, Masini E, Cuzzocrea S, Salvemini D. Modulation of prostaglandin biosynthesis by nitric oxide and nitric oxide donors. Pharmacol Rev. 2005;57:217–252. doi: 10.1124/pr.57.2.1. [DOI] [PubMed] [Google Scholar]
- Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. New Eng J Med. 2001;345:1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- Salvemini D, Misko TP, Masferrer JL, Seibert K, Currie MG, Needleman P. Nitric oxide activates cyclooxygenase enzymes. Proc Natl Acad Sci USA. 1993;90:7240–7244. doi: 10.1073/pnas.90.15.7240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvemini D, Manning PT, Zweifel BS, Seibert K, Connor J, Currie MG, et al. Dual inhibition of nitric oxide and prostaglandin production contributes to the antiinflammatory properties of nitric oxide synthase inhibitors. J Clin Invest. 1995;96:301–308. doi: 10.1172/JCI118035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber E, Harshman K, Kemler I, Malipiero U, Schaffner W, Fontana A. Astrocytes and glioblastoma cells express novel octamer-DNA binding proteins distinct from the ubiquitous Oct-1 and B cell type Oct-2 proteins. Nucleic Acids Res. 1990;18:5495–5503. doi: 10.1093/nar/18.18.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shishodia S, Koul D, Aggarwal BB. Cyclooxygenase (COX)-2 inhibitor celecoxib abrogates TNF-induced NF-kappa B activation through inhibition of activation of I kappa B alpha kinase and Akt in human non-small cell lung carcinoma: correlation with suppression of COX-2 synthesis. J Immunol. 2004;173:2011–2022. doi: 10.4049/jimmunol.173.3.2011. [DOI] [PubMed] [Google Scholar]
- Strub A, Ulrich WR, Hesslinger C, Eltze M, Fuchss T, Strassner J, et al. The novel imidazopyridine 2-[2-(4-methoxy-pyridin-2-yl)-ethyl]-3H-imidazo[4,5-b]pyridine (BYK191023) is a highly selective inhibitor of the inducible nitric-oxide synthase. Mol Pharmacol. 2006;69:328–337. doi: 10.1124/mol.105.017087. [DOI] [PubMed] [Google Scholar]
- Szabo C, Thiemermann C. Invited opinion: role of nitric oxide in hemorrhagic, traumatic, and anaphylactic shock and thermal injury. Shock. 1994;2:145–155. [PubMed] [Google Scholar]
- Ukil A, Maity S, Das PK. Protection from experimental colitis by theaflavin-3,3′-digallate correlates with inhibition of IKK and NF-kappaB activation. Br J Pharmacol. 2006;149:121–131. doi: 10.1038/sj.bjp.0706847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uno K, Iuchi Y, Fujii J, Sugata H, Iijima K, Kato K, et al. In vivo study on cross talk between inducible nitric-oxide synthase and cyclooxygenase in rat gastric mucosa: effect of cyclooxygenase activity on nitric oxide production. J Pharmacol Exp Ther. 2004;309:995–1002. doi: 10.1124/jpet.103.061283. [DOI] [PubMed] [Google Scholar]
- Vane JR, Mitchell JA, Appleton I, Tomlinson A, Bishop-Bailey D, Croxtall J, et al. Inducible isoforms of cyclooxygenase and nitric-oxide synthase in inflammation. Proc Natl Acad Sci USA. 1994;91:2046–2050. doi: 10.1073/pnas.91.6.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CC, Chen SJ, Szabo C, Thiemermann C, Vane JR. Aminoguanidine attenuates the delayed circulatory failure and improves survival in rodent models of endotoxic shock. Br J Pharmacol. 1995;114:1666–1672. doi: 10.1111/j.1476-5381.1995.tb14955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu KK, Liou JY, Cieslik K. Transcriptional Control of COX-2 via C/EBPbeta. Arterioscl Throm Vas Biol. 2005;25:679–685. doi: 10.1161/01.ATV.0000157899.35660.61. [DOI] [PubMed] [Google Scholar]
- Zamora R, Vodovotz Y, Billiar TR. Inducible nitric oxide synthase and inflammatory diseases. Mol Med. 2000;6:347–373. [PMC free article] [PubMed] [Google Scholar]


