Abstract
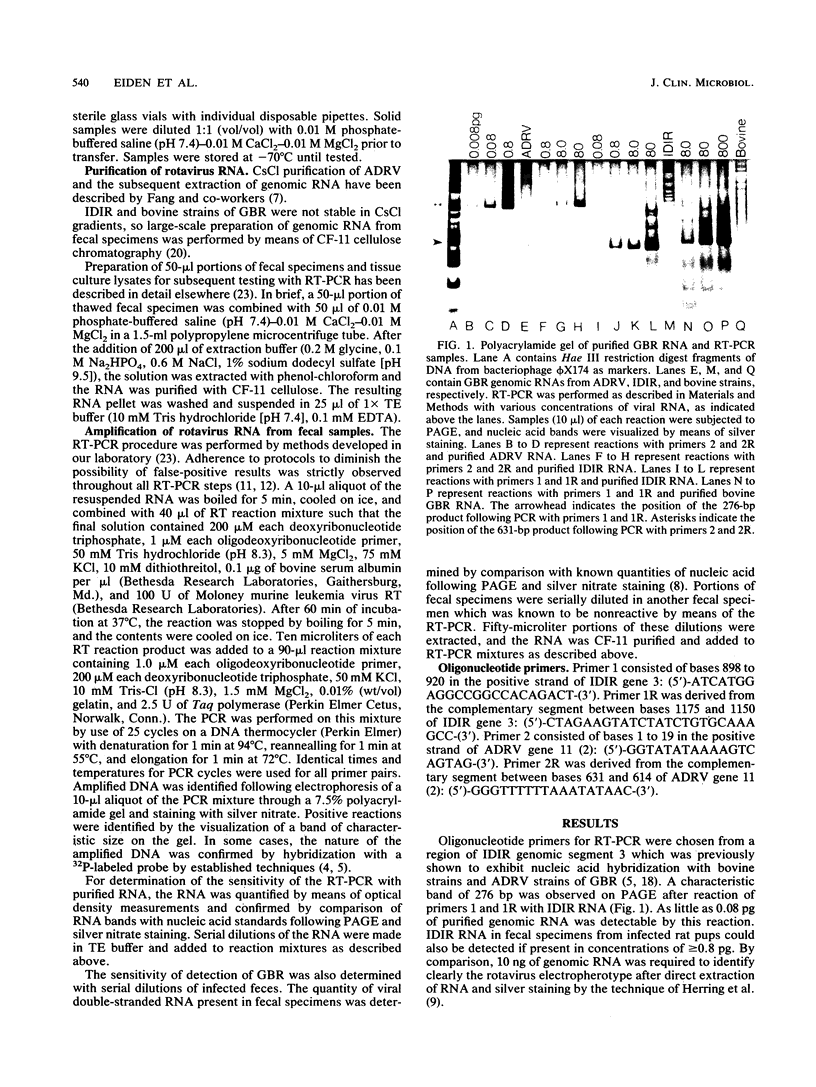
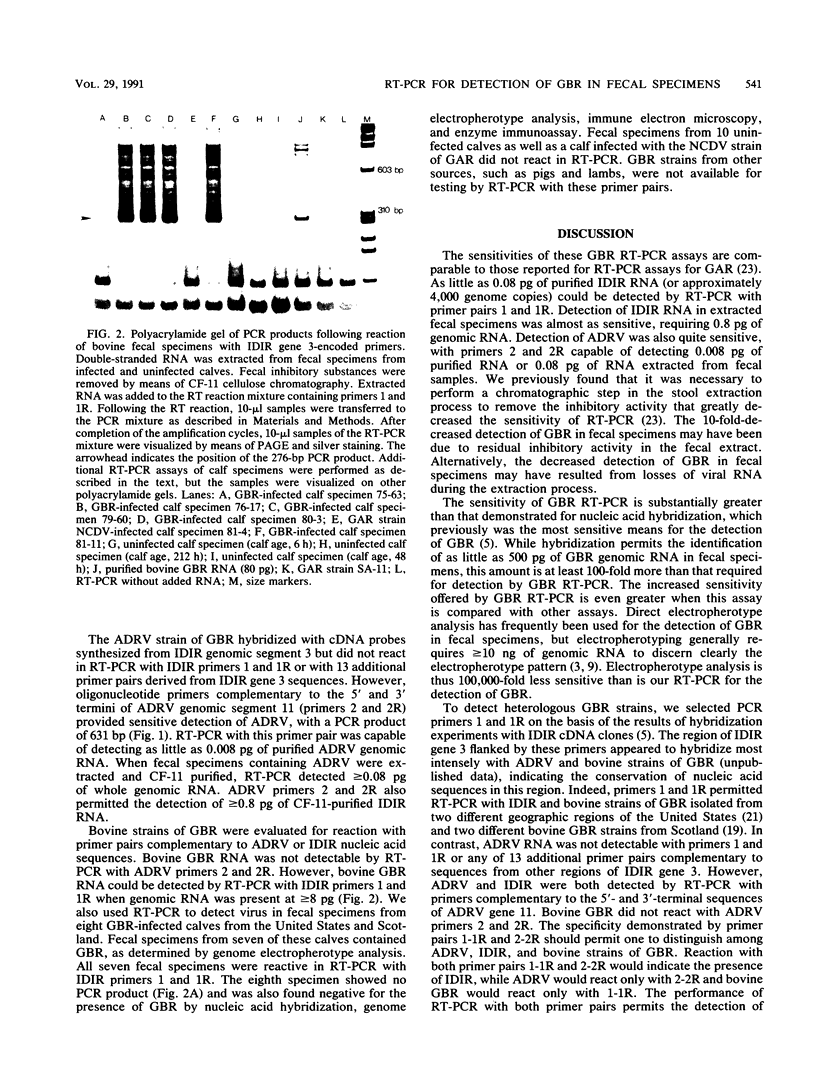
A combined reverse transcriptase reaction-polymerase chain reaction (RT-PCR) was developed to achieve the sensitive detection of group B rotaviruses (GBR). Sequences derived from genomic segment 3 of the IDIR (intestinal disease of infant rats) strain of GBR permitted the detection of greater than or equal to 0.08 pg of purified IDIR genomic RNA (4,000 genome copies). Primers complementary to the terminal sequences of gene 11 of GBR strain ADRV (adult diarrhea rotavirus) allowed for the detection of as little as 0.008 pg of purified ADRV genomic RNA. Detection of heterologous strains of GBR was also observed with these primer pairs. IDIR gene 3 primers recognized greater than or equal to 8 pg of RNA from bovine GBR obtained from a variety of geographic locations. RNA from IDIR, but not bovine GBR, strains was detected by means of RT-PCR with ADRV gene 11 primers. Neither set of GBR primers was reactive in RT-PCR with fecal specimens containing group A rotaviruses or fecal specimens from uninfected controls. This RT-PCR assay permits the sensitive and specific detection of a variety of GBR in fecal specimens.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bridger J. C. Novel rotaviruses in animals and man. Ciba Found Symp. 1987;128:5–23. doi: 10.1002/9780470513460.ch2. [DOI] [PubMed] [Google Scholar]
- Chen G. M., Hung T., Mackow E. R. cDNA cloning of each genomic segment of the group B rotavirus ADRV: molecular characterization of the 11th RNA segment. Virology. 1990 Apr;175(2):605–609. doi: 10.1016/0042-6822(90)90450-6. [DOI] [PubMed] [Google Scholar]
- Dolan K. T., Twist E. M., Horton-Slight P., Forrer C., Bell L. M., Jr, Plotkin S. A., Clark H. F. Epidemiology of rotavirus electropherotypes determined by a simplified diagnostic technique with RNA analysis. J Clin Microbiol. 1985 May;21(5):753–758. doi: 10.1128/jcm.21.5.753-758.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiden J. J., Firoozmand F., Sato S., Vonderfecht S. L., Yin F. Z., Yolken R. H. Detection of group B rotavirus in fecal specimens by dot hybridization with a cloned cDNA probe. J Clin Microbiol. 1989 Mar;27(3):422–426. doi: 10.1128/jcm.27.3.422-426.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiden J., Vonderfecht S., Theil K., Torres-Medina A., Yolken R. H. Genetic and antigenic relatedness of human and animal strains of antigenically distinct rotaviruses. J Infect Dis. 1986 Dec;154(6):972–982. doi: 10.1093/infdis/154.6.972. [DOI] [PubMed] [Google Scholar]
- Eiden J., Vonderfecht S., Yolken R. H. Evidence that a novel rotavirus-like agent of rats can cause gastroenteritis in man. Lancet. 1985 Jul 6;2(8445):8–11. doi: 10.1016/s0140-6736(85)90057-1. [DOI] [PubMed] [Google Scholar]
- Fang Z. Y., Glass R. I., Penaranda M., Dong H., Monroe S. S., Wen L., Estes M. K., Eiden J., Yolken R. H., Saif L. Purification and characterization of adult diarrhea rotavirus: identification of viral structural proteins. J Virol. 1989 May;63(5):2191–2197. doi: 10.1128/jvi.63.5.2191-2197.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herring A. J., Inglis N. F., Ojeh C. K., Snodgrass D. R., Menzies J. D. Rapid diagnosis of rotavirus infection by direct detection of viral nucleic acid in silver-stained polyacrylamide gels. J Clin Microbiol. 1982 Sep;16(3):473–477. doi: 10.1128/jcm.16.3.473-477.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung T., Chen G. M., Wang C. G., Yao H. L., Fang Z. Y., Chao T. X., Chou Z. Y., Ye W., Chang X. J., Den S. S. Waterborne outbreak of rotavirus diarrhoea in adults in China caused by a novel rotavirus. Lancet. 1984 May 26;1(8387):1139–1142. [PubMed] [Google Scholar]
- Kitchin P. A., Szotyori Z., Fromholc C., Almond N. Avoidance of PCR false positives [corrected]. Nature. 1990 Mar 15;344(6263):201–201. doi: 10.1038/344201a0. [DOI] [PubMed] [Google Scholar]
- Kwok S., Higuchi R. Avoiding false positives with PCR. Nature. 1989 May 18;339(6221):237–238. doi: 10.1038/339237a0. [DOI] [PubMed] [Google Scholar]
- Kwok S., Kellogg D. E., McKinney N., Spasic D., Goda L., Levenson C., Sninsky J. J. Effects of primer-template mismatches on the polymerase chain reaction: human immunodeficiency virus type 1 model studies. Nucleic Acids Res. 1990 Feb 25;18(4):999–1005. doi: 10.1093/nar/18.4.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakata S., Estes M. K., Graham D. Y., Loosle R., Tao H., Wang S. H., Saif L. J., Melnick J. L. Antigenic characterization and ELISA detection of adult diarrhea rotaviruses. J Infect Dis. 1986 Sep;154(3):448–455. doi: 10.1093/infdis/154.3.448. [DOI] [PubMed] [Google Scholar]
- Nakata S., Petrie B. L., Calomeni E. P., Estes M. K. Electron microscopy procedure influences detection of rotaviruses. J Clin Microbiol. 1987 Oct;25(10):1902–1906. doi: 10.1128/jcm.25.10.1902-1906.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedley S., Bridger J. C., Brown J. F., McCrae M. A. Molecular characterization of rotaviruses with distinct group antigens. J Gen Virol. 1983 Oct;64(Pt 10):2093–2101. doi: 10.1099/0022-1317-64-10-2093. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sato S., Yolken R. H., Eiden J. J. The complete nucleic acid sequence of gene segment 3 of the IDIR strain of group B rotavirus. Nucleic Acids Res. 1989 Dec 11;17(23):10113–10113. doi: 10.1093/nar/17.23.10113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snodgrass D. R., Herring A. J., Campbell I., Inglis J. M., Hargreaves F. D. Comparison of atypical rotaviruses from calves, piglets, lambs and man. J Gen Virol. 1984 May;65(Pt 5):909–914. doi: 10.1099/0022-1317-65-5-909. [DOI] [PubMed] [Google Scholar]
- Theil K. W., McCloskey C. M., Saif L. J., Redman D. R., Bohl E. H., Hancock D. D., Kohler E. M., Moorhead P. D. Rapid, simple method of preparing rotaviral double-stranded ribonucleic acid for analysis by polyacrylamide gel electrophoresis. J Clin Microbiol. 1981 Sep;14(3):273–280. doi: 10.1128/jcm.14.3.273-280.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonderfecht S. L., Eiden J. J., Torres A., Miskuff R. L., Mebus C. A., Yolken R. H. Identification of a bovine enteric syncytial virus as a nongroup A rotavirus. Am J Vet Res. 1986 Sep;47(9):1913–1918. [PubMed] [Google Scholar]
- Vonderfecht S. L., Miskuff R. L., Eiden J. J., Yolken R. H. Enzyme immunoassay inhibition assay for the detection of rat rotavirus-like agent in intestinal and fecal specimens obtained from diarrheic rats and humans. J Clin Microbiol. 1985 Nov;22(5):726–730. doi: 10.1128/jcm.22.5.726-730.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde J., Eiden J., Yolken R. Removal of inhibitory substances from human fecal specimens for detection of group A rotaviruses by reverse transcriptase and polymerase chain reactions. J Clin Microbiol. 1990 Jun;28(6):1300–1307. doi: 10.1128/jcm.28.6.1300-1307.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt R. G., James W. D., Bohl E. H., Theil K. W., Saif L. J., Kalica A. R., Greenberg H. B., Kapikian A. Z., Chanock R. M. Human rotavirus type 2: cultivation in vitro. Science. 1980 Jan 11;207(4427):189–191. doi: 10.1126/science.6243190. [DOI] [PubMed] [Google Scholar]