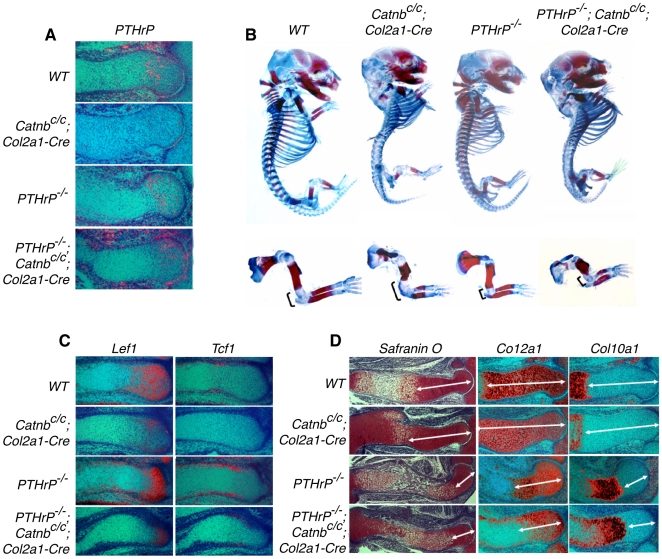
Figure 1. Analysis of PTHrP and β-catenin mutant skeletons.
(A) Sections of developing humerus at 14.5 dpc were examined by in situ hybridization with PTHrP probes. Expression of PTHrP was reduced in periarticular chondrocytes in Catnbc/c; Col2a1-Cre single mutant embryos compared to that in wild type embryos. PTHrP expression was increased in the double mutant embryos compared to that in the Catnbc/c; Col2a1-Cre single mutant embryos. (B) Skeletal preparation of 16.5 dpc embryos. A forelimb of each embryo is shown in lower panel. Alizarin red stains the mineralized hypertrophic chondrocytes and bone. Alcian blue stains the unmineralized cartilage. Mineralization was accelerated in the PTHrP−/−; Catnbc/c; Col2a1-Cre double mutant and the PTHrP−/− single mutant. The bracket indicates the non-mineralized cartilage at the joint region. (C) Consecutive sections of the radius at 14.5 dpc were examined by in situ hybridization with probes of Lef1 and Tcf1. Lef1 and Tcf1 expression were downregulated in both Catnbc/c; Col2a1-Cre single mutant and PTHrP−/−; Catnbc/c; Col2a1-Cre double mutant embryos. (D) Consecutive sections of the developing humerus at 14.5 dpc were examined by Safranin O staining and in situ hybridization with the indicated riboprobes. Safranin O staining and expression of Col2a1 marked nonhypertrophic chondrocytes. The nonhypertrophic domain (double headed arrow) was expanded in Catnbc/c; Col2a1-Cre mutant embryos whereas it was shortened to the same degree in PTHrP−/− single mutant and PTHrP−/−; Catnbc/c; Col2a1-Cre double mutant embryos. Col10a1 expression marked hypertrophic region, which was accelerated to the same level in PTHrP−/− single mutant and PTHrP−/−; Catnbc/c; Col2a1-Cre double mutant embryos.