Abstract
Objective
To investigate the correlation between radiologic vascular dilatation and serum nitrite concentration and eNOS expression in the endothelial cell and pneumocyte in a rabbit model of hepatopulmonary syndrome induced by common bile duct ligation (CBDL).
Materials and Methods
Thin-section CT scans of the lung and pulmonary angiography were obtained 3 weeks after CBDL (n=6), or a sham operation (n=4), and intrapulmonary vasodilatation was assessed. The diameter and tortuosity of peripheral vessels in the right lower lobe by thin-section CT and angiography at the same level of the right lower lobe in all subjects were correlated to serum nitrite concentration and eNOS (endothelial nitric oxide synthase) expression as determined by immunostaining.
Results
The diameters of pulmonary vessels on thin-section CT were well correlated with nitrite concentrations in serum (r = 0.92, p < 0.001). Dilated pulmonary vessels were significantly correlated with an increased eNOS expression (r = 0.94, p < 0.0001), and the severity of pulmonary vessel tortuosity was found to be well correlated with serum nitrite concentration (r = 0.90, p < 0.001).
Conclusion
The peripheral pulmonary vasculature in hepatopulmonary syndrome induced by CBLD was dilated on thin-section CT and on angiographs. Our findings suggest that peripheral pulmonary vascular dilatations are correlated with serum nitrite concentrations and pulmonary eNOS expression.
Keywords: Liver, injuries; Lung, angiography; Lung, CT; Lung, injuries
Hepatopulmonary syndrome consists of a triad of hepatic dysfunction and/or portal hypertension, peripheral pulmonary vascular dilatation, and hypoxemia in the absence of detectable primary cardiopulmonary disease (1-3). Although the disease pathogenesis is not precisely known, reports show that a vasoactive substance imbalance is likely to be responsible (4-9), and the leading contender seems to be nitric oxide (4-12). Increased circulating nitric oxide or nitric oxide synthase may account for the vasodilatation observed in hepatopulmonary syndrome. Moreover, the development of an animal model of hepatopulmonary syndrome, i.e., common bile duct ligation has provided us with a means of investigating the mechanism of intrapulmonary vascular dilatation (4-7, 9-14). However, most investigations have been focused on intrapulmonary vascular dilatation with respect to the microvasculature at the capillary and precapillary levels (4-6, 10-12, 14), though some authors (7, 14, 15) have documented radiologic findings of hepatopulmonary syndrome at the point of the peripheral pulmonary vasculature. However, to our knowledge no study has been undertaken in which pulmonary peripheral vessels were visualized on thin-section CT and pulmonary angiography and correlated with serum nitrite concentration and immunostainings. The aim of this study was to investigate the correlations between the radiologic finding of vascular dilatation with serum nitrite concentration and endothelial nitric oxide synthase (eNOS) expression in a rabbit model of hepatopulmonary syndrome induced by common bile duct ligation (CBDL).
MATERIALS AND METHODS
Animal Preparation and Methods
Experiments were performed on 17 New Zealand rabbits weighing 3-3.5 kg using a protocol approved by our institutional animal care and use committee. Animals were housed under conditions of controlled temperature and humidity (22+2℃/60% RH) and allowed ad libitum access to water and laboratory chow. Rabbits underwent fasting for 8 hours prior to anesthesia, and 0.5 mg of atropine (Daehan Atropine; Daehan Pharmaceutical, Seoul, Korea) was injected intramuscularly 30 minutes before operation in order to prevent hypersecretion of mucus in the bronchus. The rabbits were anesthetized by intramuscular injection of 5 mg/kg of 2% xylazine hydrochloride (Rompun; Bayer Korea, Seoul, Korea). Anesthesia was maintained by an intramuscular injection of 25 mg/kg of Ketamine hydrochloride (Ketalar; Yuhan Yanghang, Seoul, Korea).
CBDL was administered to 13 rabbits under anesthesia. After making an incision of 3 cm in the peritoneum through a subcostal approach, the common bile duct was ligated with No. 5 silk and the peritoneum was sutured. Three weeks after CBDL, cirrhotic change had been provoked providing a model of hepatopulmonary syndrome. Four control rabbits were subjected to laparatomy alone (sham operation) without CBD ligation. After CBDL or sham operation, animals were administered a daily injection of intramuscular cefotaxime at 20 mg/Kg (Cefotaxime, Korean United Co., Seoul, Korea). Seven rabbits of 13 CBDL were died during the period of the experiment: four did not recover from anesthesia and three died of ascitic fluid infections in the peritoneal cavity. Six rabbits left in CBDL group and four left in the controls.
CT examination
Three weeks after CBDL or the sham operation, thin-section CT scans were performed to detect pulmonary vascular dilatation at the lower lobe 1 cm above the diaphragm, in a steady-state condition, including a stable heart rate, under anesthesia to prevent spontaneous respiratory efforts. CT scans covering the entire thorax were obtained using a Somatom Plus Scanner (Siemens Medical System, Erlangen, Germany). The parameters used were; a 2-mm-collimation, 1-to-1 pitch, 1-sec scan time, 130 kVp, 220 mA, and a reconstruction interval of 1 mm with a window width of 1300 HU and a window level at -350 HU. Imaging was obtained with a 8.2 ratio zoom and 20-cm field-of-view.
Angiographic Examination
Rabbits were premedicated with 5 mg/kg of 2% xylazine hydrochloride and ketamine hydrochloride 25 mg/kg intramuscularly. Isoflurane (Aerane liquid, Ilsung, Seoul, Korea) 1.5%-2.5% was used to maintain general anesthesia. With a rabbit in a supine position, a surgical cut down was performed to gain access to the right jugular vein, into which a 4F Micropuncture sheath (Cook, Bloomington, IN, USA) was introduced. The tip of the sheath was positioned at the junction of the superior vena cava and right atrium. In order to obtain biochemical parameters and serum nitrite concentration, a 10 ml blood sample was obtained. Ten milliliter of ioxaglate 320 (ioxaglate sodium: ioxaglate meglumine = 1:3; Hexabrix 320; Guerbet, Paris, France; 320 mg of iodine per milliliter) was gently injected by hand in the superior vena cava 5 ml/sec speed. Digital subtraction angiography was performed at a 25 degree left posterior oblique position to inspect the right lower pulmonary artery. Digital subtraction angiographic images were obtained at a rate of 6 images per second.
Liver Function Test and Serum Nitrite Measurement
To evaluate hepatic laboratory function and to measure serum nitrite (the stable end product of nitric oxide), blood was obtained from CBD-ligated and sham-operated rabbits. Before angiography, 10 ml of blood was collected from the SVC, 8 ml of which was used for liver function testing and 2 ml to determine serum nitrite concentrations in blood. Whole blood was centrifuged at 6000 rpm for 3 min for serum preparation. The amount of nitrite in serum was measured by using the Griess reaction, by adding 100 µl of Griess reagent (1% sulfanilic acid, 0.1% naphthylethylenediamine dihydrochloride in 5% phosphoric acid) to 100 µl samples. Samples were incubated at 25℃ for 10 minutes, and optical density was measured at 550 nm (OD550) using a UV-VIS spectrophotometer (U-V-1201, Shimadzu, Kyoto, Japan). Nitrite concentrations were calculated by comparison versus OD550 standard solutions of sodium nitrite (2-100 µM).
Immunostaining
After lung removal, slices obtained from the right lower lobe were fixed in 10% neutral buffered formalin for 24 hours. Processed and paraffin embedded tissue blocks were sectioned at 4-6 µm and placed on Snowcoat X-traTM slides (Surgipath Co., Richmond, IL, U.S.A.). The sections were deparaffinized with xylene, hydrated with decreasing concentrations of ethanol, and then washed with phosphate buffered saline (PBS). To retrieve nuclear antigen, the sections were placed in Coplin jars, immersed in citrate buffer for 20 minutes and then placed in microwave for 5 minutes. The Labelled Streptavidin Biotin Technique (LSAB) detection system was used for immunostaining. Briefly, pretreated slides were incubated in normal goat serum for 30 minutes to block nonspecific binding. Primary antibody (anti-eNOS, Transduction Lab. Lexinton, KY, U.S.A.) was added and slides were incubated overnight for 2 hours at 25℃, washed with PBS for 2×10 minutes, incubated with avidin biotin complex-linked peroxidase solution, and then rinsed with PBS for 2×10 minutes. For a mixture of 3-amino-9-ethylcarbazole, 0.01% hydrogen peroxide, and Tris buffer 50 mmol/L, pH 7.2 was applied until positive stains were detected. After the PBS wash, the slides were mounted and examined under a microscope. Negative control slices were processed in the same manner but the primary antibody treatment was omitted. For general inspection of lung morphology, slices were also stained with conventional hematoxylin-eosin.
Data Analysis
In thin-section CT scans, pulmonary vessels were measured using a Vernier caliper (in millimeters to two places of decimals) by two radiologists (S.K.Y, J.W.L), blinded to the operation and all laboratory results. Three to five peripheral pulmonary vessels were taken within 1 cm of the pleural surface, and 1 cm above the diaphragm at the same level as the right lower lobe, in order to prevent pulsation artifacts, which often occur in the left lower lobe near the heart, in all subjects including the 4 controls. Each vessel of peripheral pulmonary vessels at the right lower lobe was measured at the same level with a Vernier caliper by the two radiologists, and the measurement obtained were compared. The average values of the diameter of vessels at this level were taken to determine vascular dilatation as used in the serum nitrite concentration or eNOS expression investigation.
Angiographic images were analyzed by three radiologists (K.N.L, S.K.Y, B.H.P) who were also unaware of operation details and laboratory findings. We compared the tortuosity of pulmonary vessels in the right lower lobe in pulmonary angiographs in the both ten CBDL and control rabbits. We scored tortuosity in 3 degrees (0, absent; 1, dilatation of a vessel segment; 2, obvious vessel bowing); decisions regarding findings were reached by consensus.
The degree of immunostaining for eNOS expression was graded into 3 groups; grade 0, no staining of parenchyma or vessels; grade 1, slight staining of parenchyma and vessels; and grade 2, strong staining of parenchyma and vessels, as determined by a lung pathologist.
The diameter and the degree of tortuosity of vessels were compared with the concentration of serum nitrite, the degree of immunostaining for eNOS expression, and with biochemical parameters (liver function tests (LFTs)). Data were analyzed using the student's t test, by ANOVA. Nonparametric correlation (Spearman) analysis was performed using SAS/STAT software (SAS Institute Inc., Cary, NC, USA) to determine the nature of the correlation between pulmonary vessel diameter on CT, or the degree of vessel tortuosity by angiography with serum nitrite concentration, eNOS expression, and LFT parameters. A p value of < 0.05 was taken to indicate a statistically significant difference.
Cohen's Kappa test was performed to determine interobserver concordance with respect to pulmonary vessel measurement using SAS/STAT software (SAS institute Inc., Cary, NC, U.S.A.). Weighted kappa co-efficient values were used to determine the levels of interobserver agreement, and were considered as slight at < 0.2; fair = 0.21-0.4; moderate = 0.41-0.6; substantial = 0.61-0.8; or almost perfect = 0.82-1.0.
RESULTS
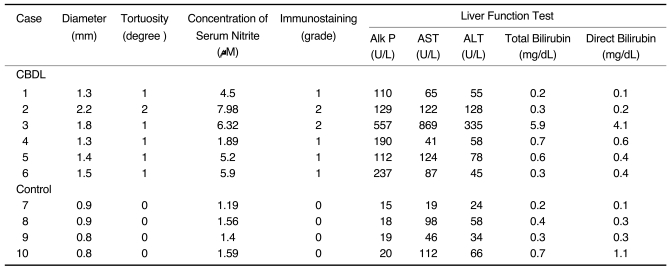
In animals three weeks after CBDL we observed dilated and tortuous pulmonary vessels. Dilated vessels ranged from 1.3 to 2.2 mm in diameter (mean diameter ± standard deviation, 1.6 mm±0.3) in the right lower lobes (Figs. 1A, 2A); in contrast, the mean diameter of vessels in control animals that underwent a sham operation was 0.9 mm±0.1 (Fig. 3A). The mean diameters and standard errors of pulmonary vessel measurements in the right lower lobes of hepatopulmonary syndrome (HPS) by CT studies as determined by readers 1 and 2 were 1.6 mm±0.2 and 1.5 mm±0.5, respectively. Mean diameters and standard errors of vessel measurements in the right lower lobes of control animals by CT as determined readers 1 and 2 were 0.8 mm±0.2 and 0.9 mm±0.1, respectively. Interobserver agreement was thus moderate in terms of pulmonary vessel measurement in HPS and in controls, with weighted kappa coefficients of 0.58 (p < 0.05) and 0.52 (p < 0.05). We found that the diameters of pulmonary vessels by CT were well correlated with both nitrite concentration in serum (r = 0.92, p < 0.001) and with an elevated eNOS expression (r = 0.94, p < 0.0001) (Table 1, Figs. 1C, 2C, 3C, 4).
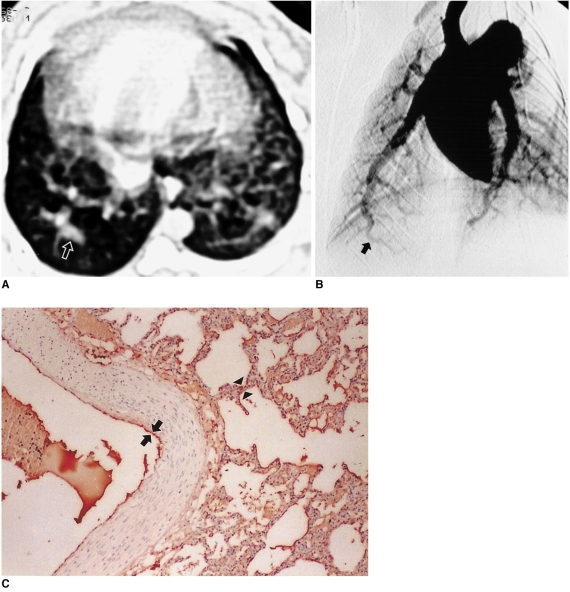
Fig. 1.
Hepatopulmonary syndrome (case 2) induced by 3 weeks after common bile duct ligation in a rabbit.
A. Transverse thin-section CT scan obtained at the lower lobe showing markedly dilated peripheral pulmonary vessel (arrow) compared with vessels at the same level in Figs. 2, 3A.
B. Pulmonary angiograph obtained at a 25 degree oblique angle showing severe tortuous right basal arteries (arrow).
C. Immunostaining for endothelial nitric oxide synthase in the right lower lobe near the level of A showing strong red immunostaining both in vascular endothelial cells (arrows) and in pneumocytes (arrowheads) (score 2).
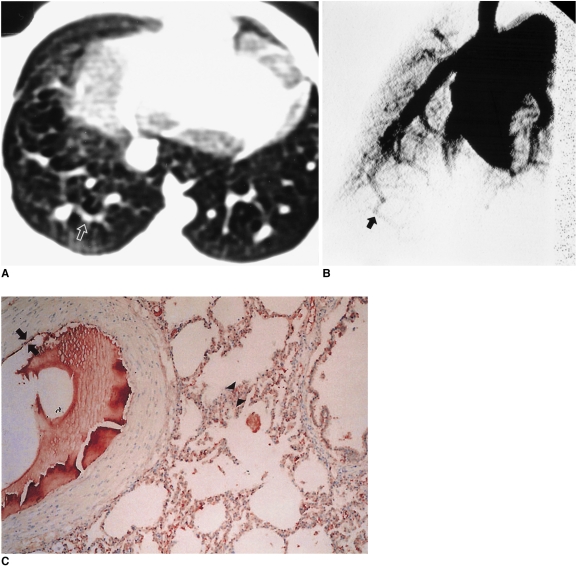
Fig. 2.
Hepatopulmonary syndrome (case 1) induced 3 weeks after common bile duct ligation in a rabbit.
A. Transverse thin-section CT scan obtained at the same level of Fig. 1A shows moderately dilated peripheral pulmonary vessels (arrow) versus vessels at the same level in Figs. 1 and 3A.
B. Pulmonary angiograph obtained at a 25 degree oblique angle showing mild tortuous right basal arteries (arrow).
C. Immunostaining for endothelial nitric oxide synthase in the right lower lobe near the level of A showing slight red immunostaining in vascular endothelial cells (arrows) and in pneumocytes (arrowheads) (score 1).
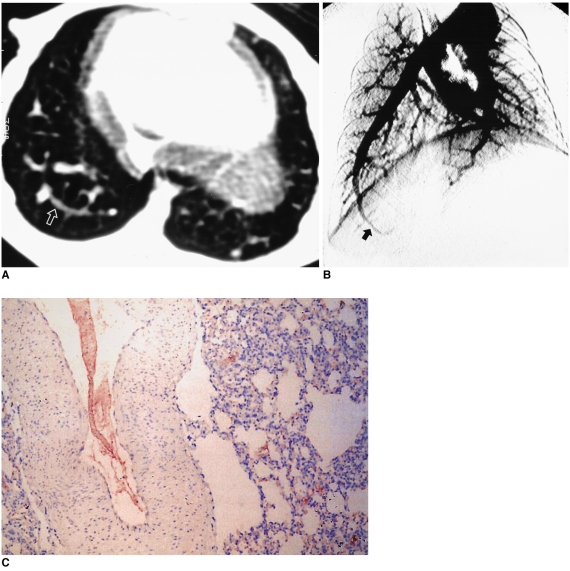
Fig. 3.
Control (case 7) - sham operation without common bile duct ligation.
A. Transverse thin-section CT scan obtained at the same level as Figs. 1 and 2A, showing no evidence of a dilated peripheral pulmonary vessel (arrow) compared with vessels at the same level in Figs. 1 and 2A.
B. Pulmonary angiographs obtained at a 25 degree oblique angle showing no evidence of tortuosity of the right basal arteries (arrow).
C. Immunostaining for endothelial nitric oxide synthase in the right lower lobe near the level of A. Vascular endothelial cells show slight immunoreactivity but no definite pneumocyte immunostaining (score 0).
Table 1.
Measurements and Tortuosity of Vessels as Seen on Thin-Section CT and Angiography, Concentration of Serum Nitrite, Immunostaining and Liver Function Test Results
Note.-CBDL = common bile duct ligation, Alk P = alkaline phosphatase, AST = aspartate transaminase, ALT = alanine transaminase.
In tortuosity, numerical degree represents 0, absence; degree 1, dilatation of a vessel segment; degree 2, obvious vessel bowing.
Immunostaining numerical grade; 0 - represents absence; grade 1 - weak; grade 2 - strong.
For SI unit conversion (µmole/L), multiply by 17.1.
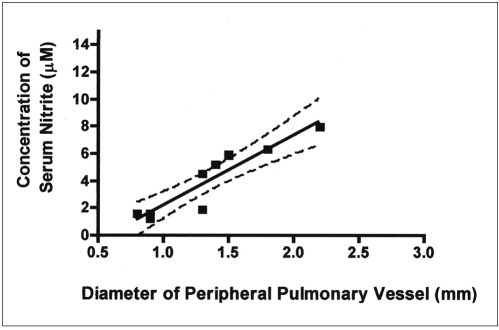
Fig. 4.
Correlation between peripheral pulmonary vessel diameter and serum nitrite concentration in 6 animals 3 weeks after common bile duct ligation and in four animals in the control group. Peripheral pulmonary vessel diameter was found to correlate significantly with serum nitrite concentration (r = 0.92, p < 0.001).
On pulmonary angiographs, right basal vessels were tortuous in HPS versus the controls (Figs. 1B, 2B, 3B), and the degree of tortuosity of pulmonary vessels was well correlated with the serum nitrite concentration (r = 0.90, p < 0.001). Serum nitrite was found to be elevated in the CBDL operated group (n = 6), rather than in the controls (n = 4) (p < 0.05).
In terms of the relationship between the degree of intrapulmonary vasodilatation to hepatic dysfunction, although laboratory findings showed hepatic dysfunction versus the controls, no correlation was observed between biochemical abnormalities and vascular dilatation or pulmonary vessels tortuosity in HPS.
DISCUSSION
The pathogenesis of intrapulmonary vascular dilatation in HPS remains an area of active investigation. In humans, enhanced pulmonary production of nitric oxide in pulmonary vasculature and in pneumocytes during the development of vasodilatation has been studied by assessing exhaled nitric oxide (17, 18). Although it is not known whether observations in CBD-ligated animals reflect human HPS, the development of an animal model of HPS offers the possibility of accelerated progress in investigations of the mechanisms of intrapulmonary vascular vasodilatation (4-12, 14). Recent work has shown that the endothelial form of nitric oxide synthase is upregulated in the pulmonary microcirculation and that this enhances nitric oxide activity and vasodilatation in affected animals (4-7, 11, 12, 14). Others have focused on alternative contributors to intrapulmonary vascular dilatation, and it was found that an unexpected potential mediator of HPS, endothelin-1, is overproduced in the CBDL liver and that it is enhanced in the systemic circulation (4-6, 12), indicating that endothlin-1 might act as an endocrine vasodilator and enhance endothelial nitric oxide production and intrapulmonary vasodilatation (4-6, 12, 14, 19).
In the present study, pulmonary vasodilatation was investigated CBDL rabbits, as a HPS model for HPS. Three weeks after CBDL, peripheral pulmonary vascular dilatation was found on thin-section CT in all HPS animals. Peripheral vessels were more dilated and tortuous by pulmonary angiography than those in the sham-operated controls, which suggests that CBDL rabbits provide a suitable model for HPS. Although a CBDL rat HPS model has shown intrapulmonary microvessel dilatation at the precapillary and capillary levels, this model conceptually appears inadequate for evaluating peripheral pulmonary vasodilatation because of the size disparity.
A recent study showed that high-resolution CT may be a less invasive radiologic method than angiography for the detection of dilated pulmonary vessels in HPS (1, 16). The degree of dilatation observed on CT was found to correlate with the severity of gas exchange abnormalities in HPS, which suggests that quantification of intrapulmonary vasodilatation is possible (1). The present study also showed a significant correlation between the degree of vasodilatation and the degree of endothelial nitric oxide synthase immunostaining, which is in agreement with the findings of other investigators (4-11, 14). In addition, the significant correlation found in the present study between intravascular dilatation and the serum concentration of nitrite, is in accord with the suggestion that nitric oxide is a major causative factor in the pathophysiological development of HPS (4-7, 11, 20).
Although pulmonary angiography is an invasive diagnostic modality for the detection of intrapulmonary vasodilatation in HPS, it may be useful for the evaluation of dilated vessel tortuosity. The present study shows tortuous vascular dilatation is also significantly related to serum nitrite concentration and eNOS expression in pulmonary vasculature and pneumocytes. Most of the rabbits with HPS after CBDL in our study showed tortuous right lower basal arteries. However, the pulmonary angiographic findings are different from published reports which mentioned a diffuse spongy form appearance in the arterial phase or even small arteriovenous communications (21, 22). We speculate that this rabbit model is inappropriate for the evaluation of small branches of peripheral pulmonary vessels, except for those of the lower basal artery.
In the present study, liver function tests showed hepatic dysfunction, but no correlation was found between biochemical abnormalities and HPS development, which concurs with the findings of studies of hepatopulmonary syndrome in man (23).
The present study has some limitations. The first being is the small number of animals involved. The second is that constitutive nitric oxide synthases like neuronal NOS (nNOS) or inducible immunologic NOS (iNOS), were not excluded as source of serum nitrite production. Considering that the immunostaining of eNOS was found in the endothelial cells of intrapulmonary vessels, some of the serum nitric oxide must have been derived from this eNOS. Thirdly, some CT scan images were blurred because of tachypnea. However, we believe that this animal model will prove useful for the evaluation of CBDL induced intrapulmonary vascular dilatation in HPS. Moreover, the application of thin-section CT to the evaluation of peripheral vessel in this study is believed to be valuable.
In conclusion, the peripheral pulmonary vasculature in this CBDL rabbit model showed dilatation by thin-section CT, tortuosity by pulmonary angiography, an increased basal nitrite production and eNOS expression, which suggest increased nitric oxide production. In summary, these results suggest that eNOS plays an important role in intrapulmonary vascular dilatation of this rabbit model of hepatopulmonary syndrome induced by common bile duct ligation.
Footnotes
This paper was supported by the Dong-A University research fund in 2003.
References
- 1.Krowka MJ. Hepatopulmonary syndrome. Gut. 2000;46:1–4. doi: 10.1136/gut.46.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Herve P, Lebrec D, Brenot F, et al. Pulmonary vascular disorders in portal hypertension. Eur Respir J. 1998;11:1153–1166. doi: 10.1183/09031936.98.11051153. [DOI] [PubMed] [Google Scholar]
- 3.Krowka MJ. Hepatopulmonary syndrome and portopulmonary hypertension: distinction and dilemmas. Hepatology. 1997;25:1282–1284. doi: 10.1002/hep.510250540. [DOI] [PubMed] [Google Scholar]
- 4.Luo B, Liu L, Tang L, Zhang J, Ling Y, Fallon B. ET-1 and TNF-alpha in HPS: analysis in prehepatic portal hypertension and biliary and nonbiliary cirrhosis in rats. Am J Physiol Gastrointest Liver Physiol. 2004;286:G294–G303. doi: 10.1152/ajpgi.00298.2003. [DOI] [PubMed] [Google Scholar]
- 5.Zhang XJ, Katsuta Y, Akimoto T, Ohsuga M, Aramaki T, Takano T. Intrapulmonary vascular dilatation and nitric oxide in hypoxemic rats with common bile duct ligation. J Hepatol. 2003;39:724–730. doi: 10.1016/s0168-8278(03)00430-6. [DOI] [PubMed] [Google Scholar]
- 6.Nunes H, Lebrec D, Mazmanian M, et al. Role of Nitric Oxide in hepatopulmonary syndrome in cirrhotic rats. Am J Respir Crit Care Med. 2001;164:879–885. doi: 10.1164/ajrccm.164.5.2009008. [DOI] [PubMed] [Google Scholar]
- 7.Lee K-N, Jung W-J, Yoon SK, et al. Correlation of pulmonary vascular dilatation on HRCT to expression of eNOS in a rabbit model of hepatopulmonary syndrome. J Korean Radiol Soc. 2002;44:475–483. [Google Scholar]
- 8.Abrams GA, Trauner M, Nathanson MH. Nitric oxide and liver disease. Gastroenterologist. 1995;3:220–233. [PubMed] [Google Scholar]
- 9.Chabot F, Mestiri H, Sabry S, et al. Role of NO in the pulmonary hyporeactivity to phenylephrine in experimental biliary cirrhosis. Eur Respir J. 1996;9:560–564. doi: 10.1183/09031936.96.09030560. [DOI] [PubMed] [Google Scholar]
- 10.Chang SW, O'Hara N. Pulmonary circulatory dysfunction in rats with biliary cirrhosis: an animal model of the hepatopulmonary syndrome. Am Rev Respir Dis. 1992;145:798–805. doi: 10.1164/ajrccm/145.4_Pt_1.798. [DOI] [PubMed] [Google Scholar]
- 11.Fallon MB, Abrams GA, Luo B, et al. The role of endothelial nitric oxide synthase in the pathogenesis of a rat model of hepatopulmonary syndrome. Gastroenterology. 1997;113:606–614. doi: 10.1053/gast.1997.v113.pm9247483. [DOI] [PubMed] [Google Scholar]
- 12.Luo B, Abrams GA, Fallon MB. Endothelin-1 in the rat bile duct ligation model of hepatopulmonary syndrome: correlation with pulmonary dysfunction. J Hepatol. 1998;29:571–578. doi: 10.1016/s0168-8278(98)80152-9. [DOI] [PubMed] [Google Scholar]
- 13.Scott VL, Dodson SF, Kang Y. The hepatopulmonary syndrome. Surg Clin N Am. 1999;79:23–41. doi: 10.1016/s0039-6109(05)70005-0. [DOI] [PubMed] [Google Scholar]
- 14.Fallon MB, Abrams GA, McGrath JW, et al. Common bile duct ligation in the rat: a model of intrapulmonary vasodilatation and hepatopulmonary syndrome. Am J Physiol. 1997;272:G779–G784. doi: 10.1152/ajpgi.1997.272.4.G779. [DOI] [PubMed] [Google Scholar]
- 15.McAdams HP, Erasmus J, Crockett R, Mitchell J, Godwin JD, McDermott VG. The hepatopulmonary syndrome; radiologic findings in 10 patients. AJR Am J Roentgenol. 1996;166:1379–1385. doi: 10.2214/ajr.166.6.8633451. [DOI] [PubMed] [Google Scholar]
- 16.Lee KN, Lee HJ, Shin WW, Webb WR. Hypoxemia and liver cirrhosis (hepatopulmonary syndrome) in eight patients: comparison of the central and peripheral pulmonary vasculature. Radiology. 1999;211:549–553. doi: 10.1148/radiology.211.2.r99ma46549. [DOI] [PubMed] [Google Scholar]
- 17.Rolla G, Brussino L, Colagrande P, et al. Exhaled nitric oxide and oxygenation abnormalities in hepatic cirrhosis. Hepatology. 1997;26:842–847. doi: 10.1053/jhep.1997.v26.pm0009328302. [DOI] [PubMed] [Google Scholar]
- 18.Rolla G, Brussino L, Colagrande P. Exhaled nitric oxide and impaired oxygenation in cirrhotic patients before and after liver transplantation. Ann Intern Med. 1998;129:375–378. doi: 10.7326/0003-4819-129-5-199809010-00005. [DOI] [PubMed] [Google Scholar]
- 19.Filep JG. Endothelial peptides: biological actions and pathophysiological significance in the lung. Life Sci. 1992;52:119–133. doi: 10.1016/0024-3205(93)90131-l. [DOI] [PubMed] [Google Scholar]
- 20.Rodriguez-Roisin R, Barbera JA. Hepatopulmonary syndrome: is NO the right answer? Gastroenterology. 1995;113:682–684. doi: 10.1053/gast.1997.v113.agast971130682. [DOI] [PubMed] [Google Scholar]
- 21.Oh KS, Bender TM, Bowen A, Ledesma-Medina J. Plain radiographic, nuclear medicine, and angiographic observations of hepatogenic pulmonary angiodysplasia. Pediatr Radiol. 1983;13:111–115. doi: 10.1007/BF01624390. [DOI] [PubMed] [Google Scholar]
- 22.Krowka MJ, Dickson ER, Cortese DA. Hepatopulmonary syndrome: clinical observations and lack of therapeutic response to somatostatin analogue. Chest. 1993;104:515–521. doi: 10.1378/chest.104.2.515. [DOI] [PubMed] [Google Scholar]
- 23.Krowka MJ. Clinical management of hepatopulmonary syndrome. Semin Liver Dis. 1993;13:414–422. doi: 10.1055/s-2007-1007369. [DOI] [PubMed] [Google Scholar]