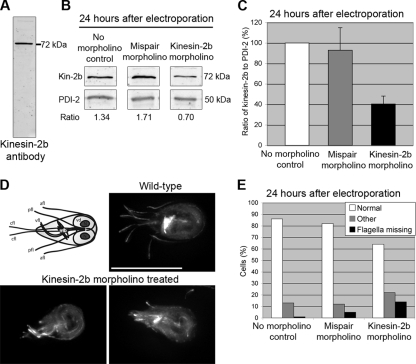
FIG. 2.
Translation-blocking morpholinos reduce GiKIN2b protein levels and cause extreme shortening of the flagella. (A) The GiKIN2b antibody produced for this study recognizes a band of the correct size (72 kDa) on Giardia extract immunoblots. (B) Results from a representative immunoblot analysis of samples 24 h after electroporation with water (no-morpholino control), 100 μM mispair anti-GiKIN2b morpholino, or 100 μM anti-GiKIN2b morpholino. PDI-2 was used as a loading control. For each sample, the ratio of GiKIN2b to PDI-2 is given. (C) Average ratios of GiKIN2b to PDI-2 on immunoblots from three experiments like the one described in the legend to panel B. The GiKIN2b/PDI-2 ratio in the no-morpholino control was set to 100%. Error bars represent one standard deviation from the average for three biological replicates. (D) Diagram of a Giardia cell and immunolabeling of wild-type and anti-GiKIN2b morpholino-treated cells. Fixed cells were labeled with an anti-α-tubulin antibody. In the diagram, the identities of the four pairs of flagella are indicated: afl, anterior flagella; pfl, posteriolateral flagella; vfl, ventral flagella; and cfl, caudal flagella. The median body (mb), a bundle of microtubules with an unknown function; the ventral disc (vd), used to attach to substrates; and two nuclei (N) are also labeled. Scale bar, 11 μm. (E) Distribution of mutant phenotypes in fixed and immunolabeled samples. Cells were collected 24 h after electroporation with water (no-morpholino control), 100 μM mispair anti-GiKIN2b morpholino, or 100 μM anti-GiKIN2b morpholino. Cells were classified as normal, mutant (missing the external portions of at least two pairs of flagella), or other (could not be categorized). One hundred cells from each sample were counted.