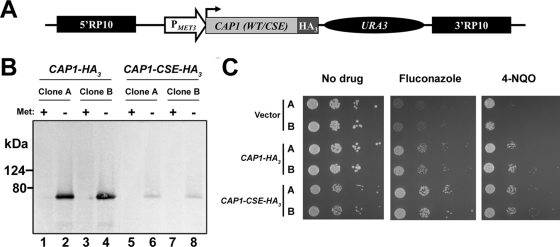
FIG. 1.
Strategy for tagging Cap1p with a HA3 epitope and characterization of the tagged strains. (A) Schematic representation of the CAP1-tagging cassette. The wild-type or mutated version of the CAP1 gene [CAP1 (WT/CSE); light gray box], cloned as an in-frame fusion with a HA3 tag (dark gray box) in plasmid pCaEXP (9), is under the control of the MET3 promoter (PMET3; open arrow) and is followed by the C. albicans URA3 marker (black oval). The 5′ and 3′ fragments of the RP10 gene (5′RP10 and 3′RP10; black boxes) flank the cassette and allow targeted integration at the RP10 locus (9). (B) Western blot analysis of strains expressing HA3-tagged versions of the CAP1 gene (CAP1-HA3 or CAP1-CSE-HA3). Total proteins were extracted from two independent clones of the SGY243 transformants (A and B) grown in the absence (−) or presence (+) of 2.5 mM methionine (Met). Western blotting was performed using the anti-HA antibody 12CA5. Positions of the molecular mass standards are indicated on the left (kDa). (C) Drug resistance profiles of C. albicans strains expressing HA3-tagged CAP1 alleles. Two independent transformants (A and B) for each of the CAP1-HA3- or CAP1-CSE-HA3-expressing strains or the strain carrying the empty vector as the negative control (vector) were analyzed by spot assay for their ability to grow on SC-ura-met plates in the absence or presence of 2 μg/ml of FLC or 1.5 μM of 4-NQO. The plates were incubated for 2 days at 30°C.