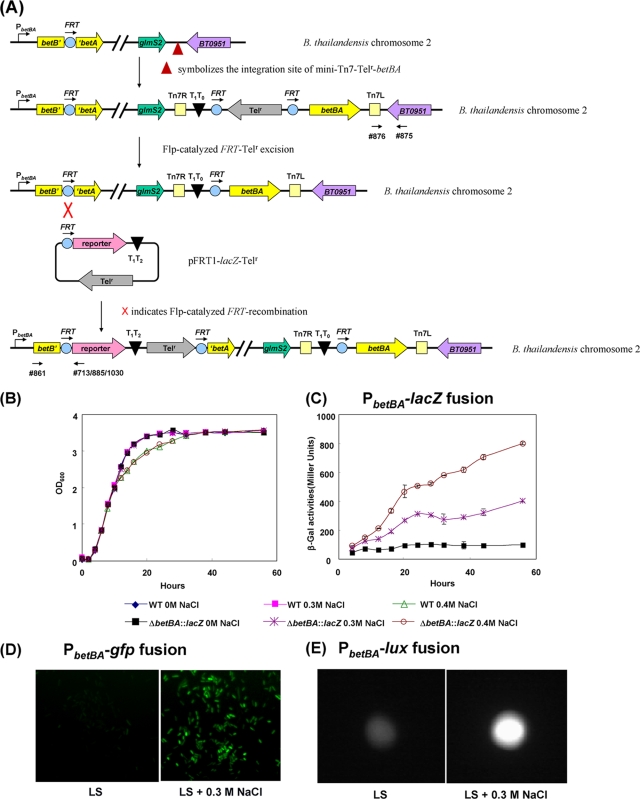
FIG. 6.
Induction of the betBA operon by osmotic stress. (A) Strategy for constructing fusion strain E264-ΔbetBA::FRT-lacZ-Telr/attTn7::betBA. Oligonucleotide 861 was used along with oligonucleotide 713, 885, or 1030 for screening, and they are indicated by arrows. Parallel diagonal lines indicate a large distance of separation on the chromosome. (B) The resulting strain, E264-ΔbetBA::FRT-lacZ-Telr/attTn7::betBA, and wild-type (WT) strain E264 were grown in LS (0 M NaCl) medium supplemented with 0.3 and 0.4 M NaCl. Both the wild-type and fusion strains produced identical growth characteristics in LS medium and LS medium plus 0.3 M NaCl yet displayed slightly decreased levels of growth in 0.4 M NaCl after mid-log phase. (C) At each time point, β-galactosidase assays were performed in triplicate on fusion strain E264-ΔbetBA::FRT-lacZ-Telr/attTn7::betBA grown in LS medium with 0, 0.3, or 0.4 M NaCl. When the complemented fusion strain was grown under osmotically stressed conditions (0.3 and 0.4 M NaCl), the betBA operon was significantly induced, compared to conditions with no osmotic stress (no salt). (D and E) Two alternative fusion strains, E264-ΔbetBA::FRT-gfp-Telr/attTn7::betBA (D) and E264-ΔbetBA::FRT-lux-Telr/attTn7::betBA (E), were grown in LS medium or LS medium plus 0.3 M NaCl. The expressions of GFP (D) and luxCDABE (E) were significantly induced in the presence of NaCl. Images in D are representative of multiple fields in the same sample. OD600, optical density at 600 nm.