Figure 2.
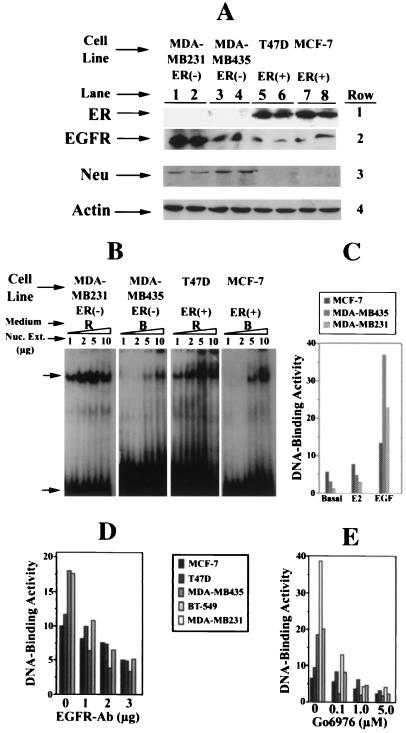
Levels of ER and EGFR family receptors and activation and inhibition of NF-κB in breast cancer cells. A shows the levels of ER, EGFR, Neu, and actin proteins in whole-cell extracts of ER− MDA-MB435 and MDA-MB231 and ER+ T47D and MCF-7 breast cancer cells in culture, as measured by Western blot analysis. Cells were grown in rich (R) medium to 90–95% confluency, whole-cell extracts were prepared (6), and 50 μg of protein in samples (in duplicate, designated by numerals under each cell line) was subjected to Western blot analysis and immunodetected with anti-ER-antibody Sc-543 (row 1) (42). The same blot was stripped and reused for detection of EGFR with anti-EGFR antibody Sc-03-G (row 2). Row 3 shows the level of Neu detected similarly with the anti-Neu antibody (Sc 284 G), and row 4 shows the levels of actin in the same samples as determined by reprobing the same blot with antiactin antibody, which serves as a loading control. These determinations were made three times, and results of one experiment are shown here. B shows the level of 32P-DNA-binding activity of NF-κB in the indicated amounts (protein) of nuclear extracts from ER− MDA-MB231 and ER+ MCF-7 cells grown in rich medium (R) or basal medium (B), as measured by EMSA (38–39). The retarded specific NF-κB-32P-DNA complex is indicated by the upper arrow, and the free 32P-DNA (NF-κB–oligonucleotide) is indicated by the bottom arrow. C shows stimulation of NF-κB-32P-DNA-binding activity by E2 and EGF. The binding activity (numerals on the y axis) represents integrated intensity of the autoradiographic signals quantitated, as described in Materials and Methods. The ER− MDA-MB231 and MDA-MB435 and ER+ MCF-7 cells were plated in 25 ml of rich medium in 150-mm tissue culture dishes. Forty-eight hours later, the medium was removed, and cells were washed with basal medium (B) and replenished with 25 ml of the same medium. Seventy-two hours later, the medium was removed and replenished with 25 ml of basal medium, and cells were grown for an additional 12 h in the presence of either E2 (10−6 M) or EGF (12 ng/ml). Nuclear extracts from the treated and control cells were prepared (40), and NF-κB-32P-DNA-binding activities in 5 μg of nuclear extracts of these samples were measured by EMSA. One of four such experiments is reported here. D shows the NF-κB-32P-DNA-binding activity in nuclear extracts (5 μg) from the four breast cancer cell lines grown in basal medium plus EGF (12 ng/m) and indicated amounts of anti-EGFR-antibody per 10 ml of basal medium for 12 h. Growth and treatment conditions of the cells were the same as described in C. NF-κB-32P-DNA-binding activity was determined in nuclear extracts from two ER− MDA-MB-435 and MDA-MB231 and two ER+, MCF-7, T47D cells by EMSA and quantitated, as described above. E shows similar analysis for the determination of NF-κB-32P-DNA-binding activity in nuclear extracts of cells treated with indicated concentrations of G06976. Growth and treatment conditions of cells are the same as described in C. Nuclear extracts from three ER− and two ER+ cells were prepared and subjected to EMSA. Quantitation of the autoradiographic signals of the NF-κB-32P-DNA complex was the same as described above.