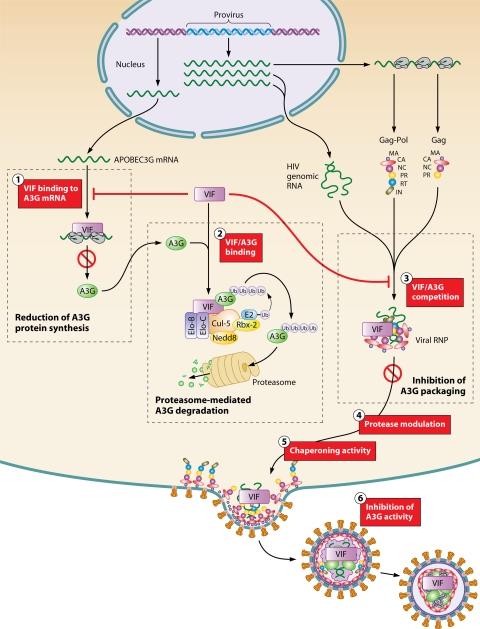
FIG. 3.
Schematic representation of Vif and hA3G functions in HIV-1 assembly and replication. During viral particle production, Vif is found in the cytoplasm of infected cells and is encapsidated in small amounts into virions. Vif neutralizes hA3G and hA3F in virus-producing cells by different mechanisms. (1) Vif has been shown to impair the translation of hA3G mRNA, probably through an mRNA-binding mechanism. (2) Vif binding to hA3G protein recruits an E3 ubiquitin ligase that mediates the polyubiquitylation of hA3G and its degradation. (3) Vif competes with hA3G for binding to viral components like the nucleocapsid domain of Gag and/or viral genomic RNA. Taken together, these three different actions of Vif on translation, degradation, and packaging not only deplete hA3G from virus-producing cells but also prevent hA3G from being incorporated in virions. (4 and 5) Intracellular Vif may also influence viral assembly through the modulation of viral protease-mediated cleavage of Gag precursors (4) and its chaperoning activities (5), thus allowing late events such as precursor maturation, initiation of reverse transcription, and RNA dimer maturation to occur after viral budding. (6) Finally, Vif might be able to directly inhibit the activity of the few hA3G molecules that are packaged in WT virions.