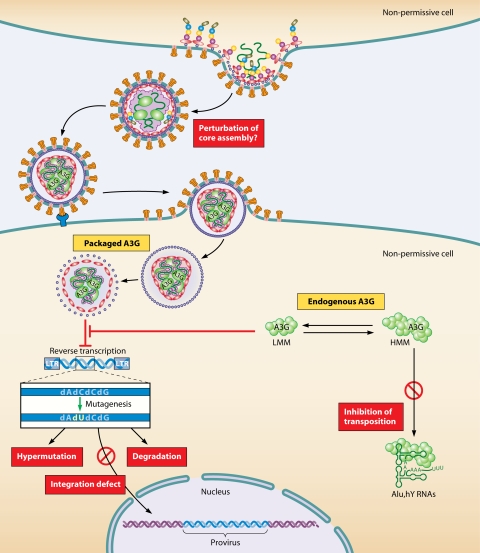
FIG. 4.
Multiple antiviral mechanisms of virion-incorporated and endogenous hA3G. In the absence of Vif, hA3G is efficiently incorporated into budding viruses. After liberation of the viral capsid into a new (nonpermissive) target cell, hA3G impedes reverse transcription and integration in a deaminase-independent antiviral action, probably through its RNA-binding properties. Once reverse transcription has started, hA3G mediates the extensive deamination of dC to dU during minus-strand DNA synthesis. This enzymatic reaction blocks HIV-1 replication due to (i) the accumulation of dG-to-dA hypermutations in the synthesized plus-stranded viral DNA (giving rise to aberrant proteins) and (ii) the degradation of viral DNA, because the U-rich DNA can be recognized by the cellular repair machinery (uracil DNA glycosylase and apurinic-apyrimidic endonuclease). Endogenous h3AG also functions as a potent restriction factor for HIV-1 in resting CD4+ T cells. hA3G exist as HMM and LMM complexes. Only the LMM complexes function as a postentry restriction factor by blocking the accumulation of reverse transcripts of incoming viruses. HMM complexes impair the transposition of Alu elements by sequestering Alu RNA transcripts away from the nuclear transposition machinery.