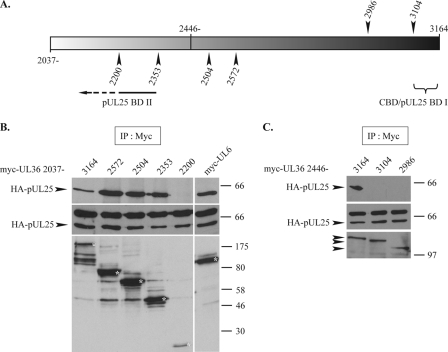
FIG. 9.
Mapping of pUL25-binding domains of pUL36. (A) Representation of the pUL36 fragment of amino acids 2037 to 3164. The arrowheads shown above mark the last amino acids encoded by the C-terminal truncations for myc-pUL36 2446, while those below are for myc-pUL36 2037. CBD/pUL25 BD I and pUL25 BD II indicate the pUL25-binding domains (pUL25 BD I is equivalent to the previously mapped capsid-binding domain) (9). (B and C) Interaction of HA-pUL25 with C-terminal truncations of myc-pUL36 2037 and myc-pUL36 2446. Vero cells were cotransfected with plasmids expressing HA-pUL25 and either full-length myc-pUL36 2037 or C-terminal truncations of myc-pUL36 2037 (B) or full-length myc-pUL36 2446 or C-terminal truncations of myc-pUL36 2446 (C), as specified above (A). Twenty-four hours after transfection, the cells were harvested, and extracts were immunoprecipitated (IP) with anti-myc antibody A14. Cell extracts (middle) and immune complexes (top) were separated by SDS-PAGE and analyzed for the presence of HA-pUL25 by Western blotting using anti-HA MAb F7. The immunoprecipitate blots were then stripped and reprobed with anti-myc MAb 9E10 to reveal the presence of the myc-pUL36 truncation products (bottom). The positions of the HA-pUL25 bands and of protein size standards are indicated to the left and right, respectively. Control extracts from cells coexpressing HA-pUL25 and myc-pUL6 were immunoprecipitated using the same procedure. The full-length myc-pUL36 truncation products and the myc-pUL6 band are marked with asterisks.