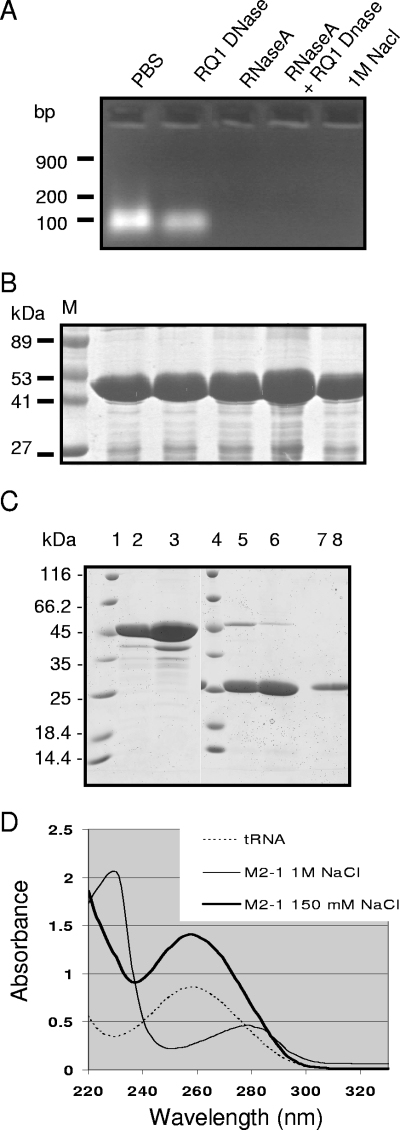
FIG. 1.
Purification of M2-1 from E. coli and analysis of the presence of nucleic acids. (A and B) Agarose gel electrophoresis analysis of nucleic acids present in the GST-M2-1 complexes (A) and SDS-PAGE analysis of GST-M2-1 complexes from the same samples (B). GST-M2-1 fusion proteins from standard bacterial lysates containing 150 mM NaCl were purified by using glutathione-Sepharose beads, resuspended in an equal volume of PBS, and then treated with RNase A, RQ1 DNase, or both or washed with 1 M NaCl, as indicated. Lane M, molecular mass markers. (C) SDS-PAGE analysis of M2-1 purified from standard bacterial lysates (lanes 2, 5, and 7) or high-salt lysates (lanes 3, 6, and 8). Proteins were cleaved by thrombin, and the pellets (lanes 5 and 6) or soluble fractions (lanes 7 and 8) were analyzed in parallel with uncleaved GST-M2-1 adsorbed on glutathione-Sepharose beads (lanes 2 and 3; 1 μl of beads per lane). Lanes 1 and 4, molecular mass markers. (D) UV spectra of tRNA and M2-1 purified from standard (150 mM NaCl) or high-salt (1 M NaCl) bacterial lysates, as indicated.