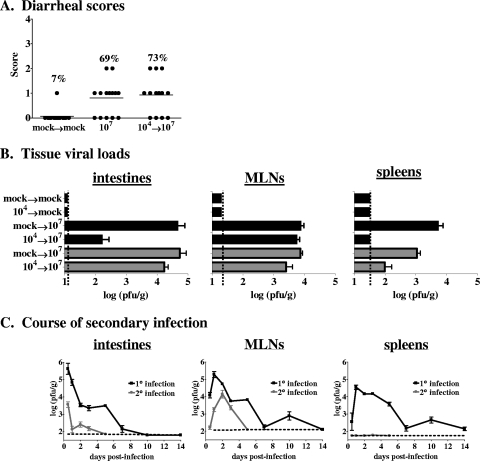
FIG. 3.
Primary MNV-1 infection does not protect from a secondary challenge. (A) Groups of 129SvEv mice (14 or 15 per group, 5 to 6 weeks of age) were inoculated perorally with 104 PFU MNV-1 or mock infected. Six weeks later, the mock-infected mice were rechallenged with a mock inoculum (mock→mock) while the MNV-1-infected mice were challenged with 107 PFU MNV-1 (104→107). For stool consistency measurement, all feces below the cecum was collected at 72 hpi and scored as described in the legend to Fig. 1. Data from 107-PFU primary infections from Fig. 1 are included for clarity (107). The frequency of mice receiving a score of 1 or greater is listed above each data set. The P value comparing MNV-1-rechallenged mice to mock-infected mice is 0.0002. (B) Groups of 129SvEv (black bars) or C57BL/6 (gray bars) mice were mock infected or inoculated perorally with 104 PFU MNV-1 (listed first on the y axis for each bar) at 5 to 6 weeks of age. Six weeks later, these same mice were either mock infected or infected with 107 PFU MNV-1 (listed second on the y axis for each bar). Two days after the secondary challenge, animals were perfused, organs were harvested, and viral burdens were determined by plaque assay. Each group contained 3 to 10 mice, and the data for all of the mice in each group were averaged. Limits of detection are indicated by dashed lines. (C) 129SvEv mice were inoculated perorally with a mock inoculum (1° infection) or 104 PFU MNV-1 (2° infection). Six weeks later, both groups of mice were inoculated perorally with 107 PFU MNV-1. At the postinfection times indicated, a minimum of three mice from each group were perfused, organs were harvested, and viral burdens were determined by plaque assay.