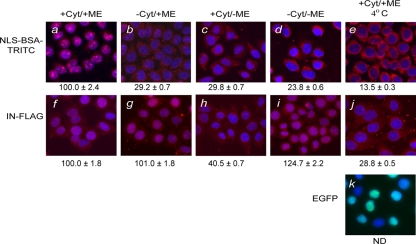
FIG. 1.
The nuclear import of HIV-1 IN is independent of cytosolic factors and metabolic energy. Nuclear import requirements of NLS-BSA-TRITC (a to e) and FLAG-tagged IN (f to j) were characterized using the semipermeabilized cell assay in the presence (+) or absence (−) of cytosolic extract (Cyt) and metabolic energy (ME) at 30°C (a to d and f to i) or at 4°C (e, j, and k). Quantitation for the level of nuclear import observed under each reaction condition was based on ≥50 nuclei. Complete reactions for NLS-BSA-TRITC (a) and HIV-1 IN (f) were set to 100%, and the levels of nuclear import observed (b to e and g to j) were calculated as percentages of the corresponding complete reactions in NLS-BSA-TRITC (a) and IN (f), respectively. Values indicated below the images are the means ± SEM for three experiments and represent the total fluorescence emanating from the import protein localized to the nucleus. NLS-BSA-TRITC was visualized directly by fluorescence microscopy. FLAG-tagged HIV-1 IN was visualized by indirect immunofluorescence using a monoclonal antibody directed at the FLAG epitope, followed by a fluorescently labeled secondary antibody. For uniformity, the fluorescence signal of both HIV-1 IN and NLS-BSA-TRITC was pseudocolored as red, while the blue fluorescence represents the DAPI-counterstained nuclei. Purified recombinant EGFP (27 kDa) was used as a control protein for passive diffusion under import conditions that restricted active transport, and its fluorescence signal was not pseudocolored. ND, not determined.