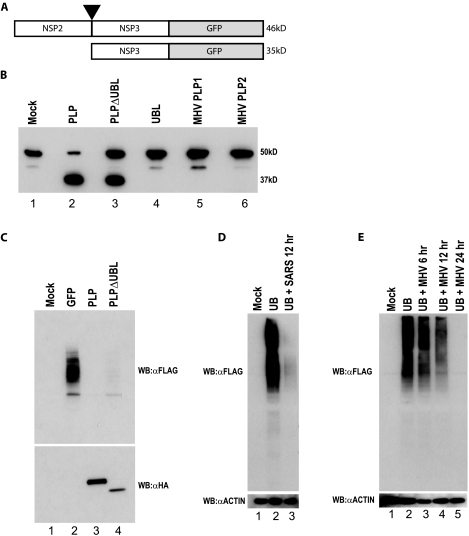
FIG. 8.
PLPΔUBL retains its protease and DUB functions. (A) A schematic of the SARS-CoV polyprotein cleavage assay is shown. The C terminal 100 aa of NSP2 through the N-terminal 80 aa of NSP3 were fused to GFP in an expression plasmid. PLP should cleave between NSP2 and -3 at the black arrowhead if the PLP protease is functional. The uncleaved product should be 46 kDa (46kD), and the cleaved product should be 35 kDa (35kD) when assayed on an SDS-PAGE gel. (B) 293T cells were transfected with either the cleavage reporter alone (mock) or the reporter and each plasmid noted above the Western blot. Proteins were extracted 24 h posttransfection and analyzed by Western blotting with an anti-GFP antibody to identify the cleaved or uncleaved cleavage reporter. The mock lane (lane 1) shows the full NSP2/3/GFP reporter. The PLP lane shows the cleaved product at ∼35 kDa. (C) PLPΔUBL retains its DUB activity. 293T cells were transfected with Flag-tagged ubiquitin and either GFP, PLP, or PLPΔUBL. Proteins were extracted 24 h posttransfection and analyzed by Western blotting (WB) with either an anti-HA antibody (αHA) (bottom panel) to visualize the PLP expression or anti-Flag antibody (αFLAG) to visualize Flag-tagged ubiquitin (top panel). (D) Vero cells were transfected with Flag-tagged ubiquitin and infected with icSARS-CoV at an MOI of 5 24 h posttransfection. Cells were lysed at 12 h postinfection and assayed for anti-Flag and antiactin (αACTIN) staining by Western blotting. (E) BHK cells were transfected with Flag-tagged ubiquitin and infected with MHV-A59 at an MOI of 5 24 h posttransfection. Cells were lysed at 6, 12, and 24 h postinfection and assayed for anti-Flag and antiactin staining by Western blotting.