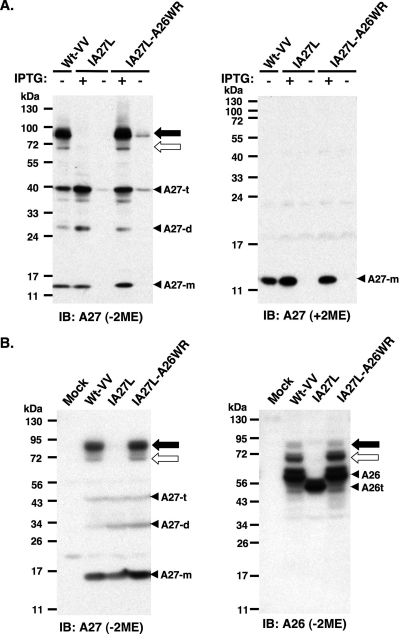
FIG. 1.
A26 and A27 proteins formed protein complexes through disulfide bond formation in BSC40 cells. (A) Immunoblot (IB) analysis of A27 protein in virus-infected cell lysates following boiling and separation by reducing (with 2ME) and nonreducing (without 2ME) SDS-PAGE. The expression of the A27L ORF in wild-type vaccinia virus (Wt-VV) was constitutive, whereas in IA27L and IA27L-A26WR viruses it was induced by 5 mM IPTG. (B) Immunoblot analysis of A27 and A26 proteins in virus-infected cells cultured in medium containing IPTG. Lysates were boiled, separated on nonreducing (without 2ME) SDS-PAGE, and probed with anti-A26 (1:1,000) and anti-A27 (1:1,000) Abs. The 90- and 70-kDa protein complexes are indicated by black and white arrows, respectively. Three protein bands representing A27 monomers (A27-m), dimers (A27-d), and trimers (A27-t) also were detected. A26t, truncated A26 protein (aa 1 to 426).