Abstract
Cancer chemotherapy drugs, such as cisplatin, potently produce nausea and vomiting. Acute effects of these treatments are partly controlled by antiemetic drugs, but the delayed effects (>24 h), especially nausea, are more difficult to treat. It is unknown what brain pathways produce this delayed sickness. Our prior data show that brain Fos expression is increased for at least 48 h after cisplatin treatment in the rat, a nonvomiting species. Here, we extend these observations by using house musk shrews (Suncus murinus), a species with an emetic response. Compared with saline injection, cisplatin treatment (30 mg/kg ip) induced Fos expression in hindbrain areas known to play a role in the generation of emesis, the dorsal motor nucleus (DMN), the area postrema, and the nucleus of the solitary tract (NTS), for up to 48 h. Cisplatin also stimulated Fos expression in the parabrachial nucleus (PBN) of the midbrain and the central nucleus of the amygdala (CeA) for at least 48 h after treatment. When animals were pretreated with the antiemetic palonosetron, a long-term serotonin type 3 (5-HT3) receptor antagonist, cisplatin-induced Fos expression was significantly attenuated in the NTS, DMN, and CeA at 6 h but not at 48 h. These results indicate that cisplatin activates a neural system that includes the dorsal vagal complex and forebrain in the musk shrew, which is partially suppressed by a 5-HT3 receptor antagonist. Our findings suggest the existence of an extensive neural system that could be targeted to reduce nausea, vomiting, and malaise in cancer patients receiving chemotherapy.
Keywords: emesis, palonosetron, serotonin type 3 receptor
nausea and vomiting are among the most common and severe side effects of chronic disease states and pharmacological treatments. Some cancer chemotherapy drugs, such as cisplatin, are extremely potent agents for producing nausea and vomiting, as well as anorexia, body weight loss, and diarrhea (22, 34, 35, 48). Chemotherapy induces both acute (up to 24 h) and delayed (>24 h) phases of nausea and emesis (57). Serotonin type 3 (5-HT3) receptor antagonists largely control the severity of chemotherapy-induced acute-phase emesis, while neurokinin 1 (NK1) receptor antagonists appear most useful for treatment of the delayed phase (16, 35, 72), although there is some overlap in their therapeutic time window. Despite the use of these first-line antiemetic drugs, many patients still experience nausea and emesis with chemotherapy, and although acute emesis is reasonably well controlled, nausea and delayed emesis are more difficult to treat (1, 57).
Several animal models have been used to study acute and delayed emesis and malaise induced by chemotherapy agents. Ferrets, pigs, dogs, and the house musk shrew (Suncus murinus) show delayed phases of emesis after injection with chemotherapy drugs (20, 43, 58, 63). Although rats, and likely all rodents, do not have a vomiting response, they do ingest kaolin clay (a form of pica), which has been used as a surrogate marker of emesis because this response appears to be a strategy to reduce toxicosis (toxins can bind to clay) and is inhibited by antiemetic drugs (5, 60, 68). Our laboratory has demonstrated (14) that rats show acute and delayed phases of kaolin intake in response to cisplatin injection. We have also shown that, similar to emesis (4), cisplatin-induced kaolin ingestion is largely dependent on an intact vagus (14) and cisplatin activates the vagus (27). In general, however, the neural systems and possible hindbrain-forebrain connections responsible for acute and delayed nausea and emesis are unknown.
The global activation of brain systems can be partly characterized by labeling Fos protein in neuronal cells after stimulation (61). Cisplatin treatment in vomiting species such as the cat and ferret produces acute Fos expression in the caudal hindbrain, including the nucleus of the solitary tract (NTS) and the area postrema (AP), areas known to play a role in emesis (7, 45, 70). The rat also shows increased c-fos mRNA expression in the NTS and the AP for up to 6 h after injection with cisplatin (18). Similarly, our laboratory has identified (26) long-term increases in Fos expression (up to 48 h) in the NTS and the AP and the amygdalar complex in rats treated with cisplatin. These data suggest a neural system that includes both the dorsal vagal complex (DVC) and forebrain areas that might be linked to the delayed phase of sickness produced by cisplatin treatment. Although our prior study defined the activation of neural pathways associated with chemotherapy-induced malaise in the rat over a 48-h period, it would be advantageous to assess activation of these neural connections with an animal model capable of vomiting.
Therefore, in the present study, we assessed the effects of cisplatin on brain Fos expression in S. murinus. The musk shrew is a well-defined model for emesis, including cisplatin-induced emesis (41, 59, 69), and shows a prominent Fos response in hindbrain emetic circuitry (6, 28, 29). We evaluated Fos expression at specific locations of the hind-, mid-, and forebrain at both acute (6 and 24 h) and long-term (48 h) time points. We chose these times because they align with sample times used in our study on cisplatin-induced Fos expression in the rat and also reflect times for acute and delayed emesis in the musk shrew (26, 62). Although we did not measure the emetic responses from animals used in this study, musk shrews from our colony do show a significant amount of emesis when injected with cisplatin. Furthermore, to address the issue of how Fos expression induced by cisplatin might relate to known emetic inputs, we used pretreatment with the antiemetic drug palonosetron to antagonize 5-HT3 receptors. We chose this specific antagonist on the basis of evidence that, unlike other clinically available 5-HT3 antagonists, palonosetron might show usefulness for the delayed phase of emesis as well as a potent effect on the acute phase and has a very long action to inhibit 5-HT3 receptors (2, 13, 16, 21).
METHODS
Animals.
We used 59 female house musk shrews (S. murinus) (34–75 days of age, 20–28 g) in this study. The musk shrews were born at the Monell Chemical Senses Center animal facility, in a colony created from stock animals obtained from Dr. Emilie Rissman at the University of Virginia (Charlottesville, VA) (9). We individually housed the musk shrews in plastic cages in a temperature-controlled vivarium on a 12:12-h light-dark cycle (lights on at 0600). All musk shrews had ad libitum access to tap water and food (75% Purina Cat Chow Complete Formula and 25% Complete Gro-Fur mink food pellets; Milk Specialty, New Holstein, WI), except as indicated below. The protocol was approved by the Monell Chemical Senses Center Institutional Animal Care and Use Committee.
Drug injections and tissue collection.
Musk shrews received two intraperitoneal injections, 15 min apart. All injections were completed between 1000 and 1145. The first injection was either saline (0.15 M NaCl) or palonosetron (0.5 mg/kg), and the second injection was either saline or cisplatin (30 mg/kg). Drugs were injected in a volume of 10 ml/kg body wt. Cisplatin (Sigma-Aldrich; cis-diamineplatium dichloride, no. P4394) and palonosetron (Haorui Pharma-Chem, Edison, NJ) were dissolved in saline (0.15 M NaCl), sonicated until clear, and then vortexed immediately before injection. Animals were randomly assigned to receive one of three drug injection combinations, saline-cisplatin, palonosetron-cisplatin, palonosetron-saline, or saline-saline. Animals receiving saline-saline were euthanized at 6 h (n = 5), 24 h (n = 6), or 48 h (n = 5) after injection. Animals receiving saline-cisplatin were euthanized at 6 h (n = 7), 24 h (n = 8), or 48 h (n = 6) after injection. Animals receiving palonosetron-cisplatin were euthanized at 6 h (n = 8) or 48 h (n = 7) after injection. An additional group receiving palonosetron-saline was euthanized at 6 h (n = 4). We removed water bottles and food jars 2 h before euthanization to minimize contributions of ingestive behavior to Fos expression.
We chose the dose of cisplatin on the basis of previous work (62) and our own data showing reliable short-term induction of emesis (unpublished data). Doses of 20–40 mg/kg cisplatin produce acute and delayed emesis in musk shrews (62). We chose the dose of palonosetron (0.5 mg/kg) based on our experiments in the rat showing that this concentration maximally suppressed 5-HT-induced electrophysiological activation of afferent vagal fibers (unpublished data) and findings that lower doses suppress cisplatin-induced acute emesis in the dog and ferret (15). Single-bolus injections of palonosetron have inhibitory effects on chemotherapy-induced acute and delayed emesis in humans, with a half-life in plasma of 44–128 h (17).
At the time of euthanasia, we deeply anesthetized the animals by intraperitoneal injection of pentobarbital sodium (25 mg; 0.5 ml). We opened the thoracic cavity with scissors and inserted a blunt-edged syringe needle into the apex of the heart. Animals were then transcardially perfused with ∼15 ml of 0.2 M phosphate-buffered saline (PBS; pH 7.4), followed by ∼20 ml of 2% acrolein-4% paraformaldehyde in 0.1 M phosphate buffer (7.4). Whole brains were removed and placed in 10% sucrose-PBS followed by 20% and 30% sucrose-PBS, each overnight. After sucrose cryoprotection, brains were frozen with dry ice and subsequently cut at 50 μm with a cryostat.
We collected sections from three locations on the basis of previous work in both the rat and the musk shrew (6, 26, 28–30): 1) the caudal hindbrain [dorsal motor nucleus (DMN), NTS, and AP], 2) midbrain sections containing the parabrachial nucleus (PBN), and 3) forebrain sections containing the central nucleus of the amygdala (CeA) and the bed nucleus of the stria terminalis, ventral (BNSTv). The PBN, CeA, and BNSTv were selected for analysis because tract tracing in the musk shrew and rat indicates that these areas have primary rostral projections from the NTS and the AP (31, 32, 65). The part of the BNST chosen for analysis is not the one represented in our paper on the rat (26), but instead is a division located ventral to the anterior commissure that shows a dense projection from the NTS of the shrew (see plate IV, section R in Ref. 31). The dorsal lateral BNST that was reported in our paper on the rat (26) was difficult to locate in the shrew brain. Sections were collected in duplicate, placed into culture plate wells containing cryoprotectant (73), and either stored at −20°C or immediately processed for immunohistochemistry.
Fos immunohistochemistry.
We processed sections with a slightly modified procedure previously established in our laboratory (26). We include here only the important modifications. Sections were incubated at room temperature in 1:10,000 polyclonal anti-Fos antibody (sc-52, Santa Cruz Biotechnology, Santa Cruz, CA; lot no. E1606) containing 1% normal donkey serum for 20 h. Biotinylated rabbit anti-rat was used as a secondary antibody (1:400, Jackson ImmunoResearch Laboratories, West Grove, PA) for 2 h at room temperature. This was followed by incubation in avidin-biotin and then diaminobenzidine (DAB)-nickel for the chromogen reaction.
Cell counting and analysis.
We determined Fos cell staining by the presence of a blue-black reaction product in the cell nuclei. Tissue sections were viewed with a Zeiss microscope (Axiostar Plus) equipped with a digital camera (Scion CFW-1312C). Brain regions and cells expressing Fos were imaged with ImageJ software (National Institutes of Health; http://rsb.info.nih.gov/ij). These images were subsequently merged with outlines of areas of interest and templates of Fluoro-Gold staining with CorelDraw (Graphics Suite X4). Subsequently, JPEG image files were generated and counted manually with ImageJ with the Measure and Label plug-in. Fos cell staining was counted by two people working independently without knowledge of the experimental conditions, and the average of these two assessments is reported here (these values had an interrater Pearson correlation of 0.90–0.97).
On the basis of previous examinations of brain sections from rats treated with cisplatin (26), we counted cells in areas that consistently showed Fos expression, with boundaries determined by reference to other brain sections containing cresyl violet or Fluoro-Gold staining (see below). To standardize the analyses, we counted cells from each area in coronal brain sections from each animal at approximately the same level (see Figs. 1 and 2). The brain areas analyzed were 1) hindbrain: NTS, AP, and DMN at +250 μm from the obex; 2) midbrain: PBN; and 3) forebrain: CeA and BNSTv. Although we show in Fig. 1 that Fos labeling produced by cisplatin did extend to rostral levels of the DVC, +400 and +700 μm rostral to the obex, we did not attempt to count these areas because it was difficult to separate the NTS from the DMN. We did not count the cells in the reticular formation since this staining was very diffuse (see Fig. 6). Fos expression was not lateralized in any of the bilateral structures examined, and cell counts reflect the totals for both sides. Brains were processed in batches, and each batch also included a positive control for Fos expression, consisting of brain sections from rats treated with cholecystokinin (100 μg/kg ip) 1 h before euthanasia (see, e.g., Ref. 50). These positive controls consistently showed high levels of Fos expression in the hindbrain and forebrain. We were also able to completely block Fos staining in samples of shrew and rat tissue by preincubation of the Fos antibody with Fos protein (sc-52P, Santa Cruz; lot no. J1805).
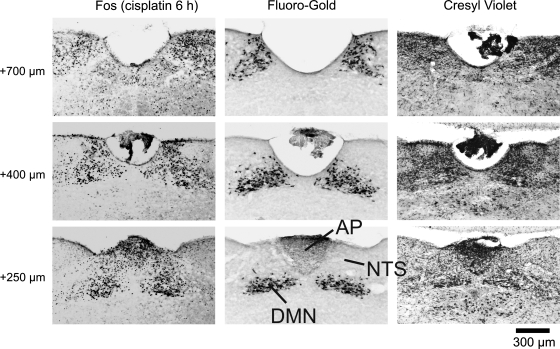
Fig. 1.
Representative images from the Suncus murinus hindbrain showing the rostral extent of Fos expression induced by cisplatin treatment in the dorsal vagal complex (DVC). Areas analyzed for Fos expression included the nucleus of the solitary tract (NTS), area postrema (AP), and dorsal motor nucleus (DMN). Six hours after cisplatin (30 mg/kg ip) treatment, Fos expression (left) was increased at several levels (+250, +400, and +700 μm) rostral to the obex. The boundaries of hindbrain areas were determined by retrograde labeling of the DMN with Fluoro-Gold (center) and cresyl violet staining of cytoarchitecture (right).
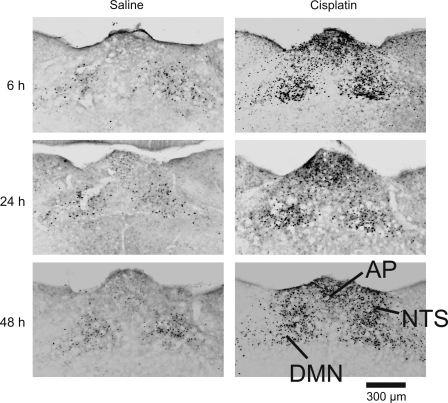
Fig. 2.
Representative images of hindbrain Fos expression at the level of the AP (+250 μm rostral to the obex) 6, 24, and 48 h after intraperitoneal injection with saline (left) or cisplatin (30 mg/kg; right).
Hindbrain, CeA, and BNSTv areas were easily identifiable in almost all of the animals; however, we could not locate appropriate levels of the DVC in 2, the CeA in 6, and the BNSTv in 10 animals. The PBN proved to be particularly problematic because we typically observed only one section at the appropriate location in each animal (see Fig. 4). Furthermore, these sections often contained only one side, left or right, because even a slight difference in the leveling of the brain during cutting would completely change the position within the PBN. Therefore, for most cases we only used one side (47% of the animals) for analysis. We doubled this value to account for the missing side to produce a more accurate Fos cell count compared with other brain areas. In 12 animals we were unable to locate an appropriate PBN section. Missing sections were distributed across the different conditions.
Cresyl violet and Fluoro-Gold staining to determine nuclear boundaries.
No detailed brain atlases are available for S. murinus, and initial observations indicated that it was difficult to determine boundaries of nuclear areas in this species. Therefore, we used cresyl violet staining to determine gross anatomic landmarks with reference to homologous areas in the rat brain and retrograde tracing to define the DMN distinctly relative to the NTS.
We stained the entire brain with cresyl violet in one musk shrew to more accurately depict the cytoarchitectural boundaries. Animal euthanization and brain tissue collection were the same as described above, except that sections (50 μm) were placed directly on slides as they were cut. Figure 1, right, shows representative images collected from the hindbrain.
In a separate experiment, two animals were injected with 0.2 ml (1% solution ip) of the retrograde tracer Fluoro-Gold (Fluorochrome, Denver, CO) to specifically identify the location of DMN neurons. This procedure has been used previously in our laboratory (14) and others (52) to define the DMN in the rat. Four to five days after injection with Fluoro-Gold animals were euthanized as described above, and 50-μm sections were processed for Fluoro-Gold immunoreactivity as described previously (14). Figure 1, middle, shows representative images collected from the hindbrain.
Statistical analysis.
Fos cell counts were analyzed by two-way analysis of variance (ANOVA) for each brain area. To assess the time effects of cisplatin, 2 (saline or cisplatin) × 3 (6, 24, or 48 h) ANOVAs were used. For the analysis of palonosetron effects, 3 (saline, cisplatin, or cisplatin + palonosetron) × 2 (6 or 48 h) ANOVAs were used. Planned comparisons were conducted with least significant difference (LSD) tests. Although it was not possible to do a full three-way ANOVA using palonsetron as a factor (i.e., time × cisplatin × palonosetron), saline-saline and palonosetron-saline groups, at 6 h, were compared with independent two-sample Student's t-tests (2-tailed). The palonosetron-saline data are included in Figs. 7 and 8 for reference. For all analyses, a P value of 0.05 was used to determine statistical significance. Group values are reported as means ± SE. Statistical analyses were conducted with Statistica (version 8, StatSoft).
RESULTS
Hindbrain Fos expression after cisplatin treatment.
Cisplatin produced both short- and long-term increases in Fos expression in the hindbrain at several levels rostral to the obex (Fig. 1). There was also a scattering of Fos expression in the reticular formation, which likely included nucleus ambiguus and C1/A1 regions, produced by cisplatin (see Fig. 6). With the use of Fluoro-Gold and cresyl violet staining (Fig. 1) to delineate nuclear boundaries, it was clear that Fos expression was elevated in the DMN, NTS, and AP at 6, 24, and 48 h after cisplatin treatment compared with saline injection (Fig. 2).
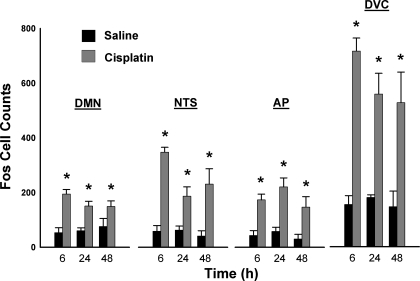
Fos cell counts (250 μm rostral to the obex) were increased in the DMN, NTS, AP, and DVC by cisplatin treatment [all F(1,30) ≥ 34.3; all P ≤ 0.00001, cisplatin main effect for each brain region; Fig. 3]. Planned comparisons showed that in all cases cisplatin treatment produced greater Fos cell counts than saline treatment (LSD tests; Fig. 3). There were no significant interaction effects for treatment by time; however, there was a significant decrease in Fos cell counts over time in the NTS [F(2,30) = 3.3; P < 0.05, main effect of time].
Fig. 3.
Fos cell counts (means ± SE) in the hindbrain 6, 24, and 48 h after intraperitoneal injection with saline or cisplatin (30 mg/kg). DVC = DMN + NTS + AP. *P < 0.05, least significant difference (LSD) test, cisplatin compared with saline injection.
Mid- and forebrain Fos expression after cisplatin treatment.
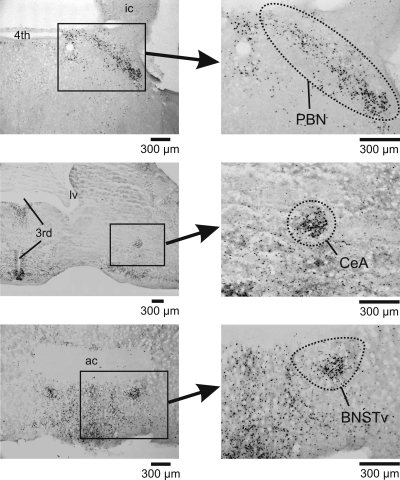
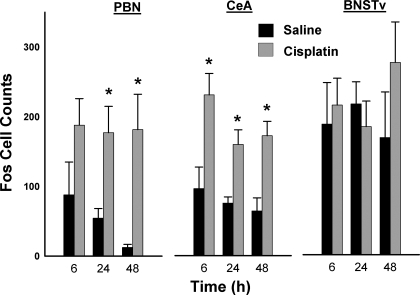
We observed scattered levels of Fos staining in the mid- and forebrain, but the PBN and the CeA showed clear effects of cisplatin treatment (Fig. 4). The PBN and the CeA showed significantly increased Fos expression after cisplatin treatment [PBN: F(1,24) = 14.3, P < 0.001; CeA: F(1,27) = 32.0, P = 0.000005; main effects of cisplatin; Fig. 5]. Planned comparisons showed that at all time points in the CeA and the PBN, except at 6 h for the PBN, cisplatin treatment produced greater Fos cell counts than saline treatment (LSD tests; Fig. 5). There were no significant interaction effects or main effects of time. Furthermore, there were no significant effects of cisplatin at any time point on Fos expression in the BNSTv (ANOVA and planned comparisons, all P > 0.05).
Fig. 4.
Representative images of midbrain and forebrain Fos expression at the parabrachial nucleus (PBN),the central nucleus of the amygdala (CeA), and the bed nucleus of the stria terminalis, ventral (BNSTv) 6 h after intraperitoneal injection with cisplatin (30 mg/kg). ic, Inferior colliculus; 4th, 4th ventricle; 3rd, 3rd ventricle; lv, lateral ventricle; ac, anterior commissure.
Fig. 5.
Fos cell counts (means ± SE) in the PBN, the CeA, and the BNSTv 6, 24, and 48 h after intraperitoneal injection with saline or cisplatin (30 mg/kg). *P < 0.05, LSD test, cisplatin compared with saline injection.
Effect of palonosetron on cisplatin-induced hindbrain Fos expression.
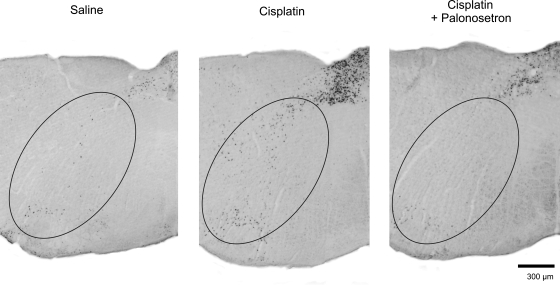
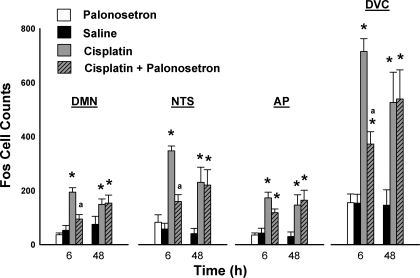
Cisplatin treatment induced Fos expression in the DMN, NTS, AP, and DVC [all F(2,30) ≥ 10.7, all P ≤ 0.001, main effects of treatment; see Fig. 7]. Treatment condition and time significantly affected Fos expression in the DMN [F(2,30) = 3.3, P < 0.05, treatment by time interaction; see Fig. 7]. There were no other significant interaction effects or main effects of time. Mean comparisons revealed that cisplatin-induced Fos expression in the DMN and the NTS was attenuated by pretreatment with palonosetron (LSD tests, P < 0.05, cisplatin vs. cisplatin plus palonosetron; see Fig. 7). The scattering of Fos expression in the reticular formation produced by cisplatin appeared to be attenuated by palonosetron (Fig. 6).
Fig. 6.
Representative images of hindbrain Fos expression in the reticular formation (oval areas) at the level of the AP (+250 μm rostral to obex, see Fig. 2) 6 h after intraperitoneal injection with saline, cisplatin (30 mg/kg), or cisplatin + palonosetron (0.5 mg/kg). Palonosetron, a serotonin type 3 (5-HT3) receptor antagonist, was injected 15 min before injection with cisplatin. Images show only one half of the hindbrain, and Fos expression in the reticular formation was present on both sides. The lower 1/3 of the region of interest likely includes the nucleus ambiguous and C1/A1.
To determine whether palonosetron might reduce basal levels of Fos expression in the hindbrain we compared saline-saline- with palonosetron-saline-treated shrews. There was no significant difference at 6 h between shrews treated with saline-saline and those treated with palonosetron-saline in the DMN, NTS, AP, or DVC [all t(7) ≤ 0.8, all P ≥ 0.5; Fig. 7].
Fig. 7.
Fos cell counts (means ± SE) in the hindbrain 6 and 48 h after intraperitoneal injection with saline or cisplatin (30 mg/kg), with and without pretreatment with palonosetron (a 5-HT3 receptor antagonist). *P < 0.05, LSD test, cisplatin or cisplatin + palonosetron compared with saline injection; aP < 0.05, LSD test, cisplatin vs. cisplatin + palonosetron.
Effect of palonosetron on cisplatin-induced mid- and forebrain Fos expression.
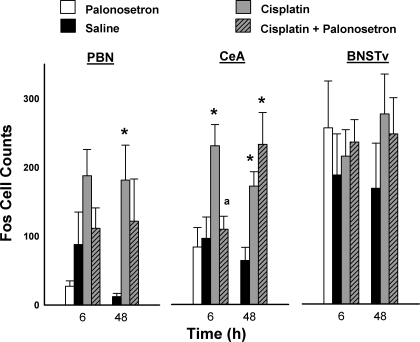
Cisplatin treatment produced Fos expression in the PBN and the CeA [PBN: F(2,23) = 4.6, P = 0.02; CeA: F(2,28) = 7.9, P = 0.002, main effects of treatment; Fig. 8]. Treatment condition and time significantly affected Fos expression in the CeA [F(2,28) = 5.9, P = 0.007, treatment by time interaction; Fig. 8]. There were no other significant interaction effects or main effects of time. In the PBN, only cisplatin at 48 h produced an increase in Fos expression (LSD test, Fig. 8). In the CeA, planned comparisons revealed that cisplatin-induced Fos expression at 48 h was attenuated by pretreatment with palonosetron (LSD test, P < 0.05, cisplatin vs. cisplatin plus palonosetron; Fig. 8). Both cisplatin and cisplatin plus palonosetron conditions produced significant increases in Fos expression in the CeA at 48 h (LSD tests; Fig. 8). In the BNSTv, there were no significant effects of cisplatin or cisplatin plus palonosetron at any time point on the level of Fos expression (ANOVA and planned comparisons, all P > 0.05).
Fig. 8.
Fos cell counts (means ± SE) in the PBN, the CeA, and the BNSTv 6 and 48 h after intraperitoneal injection with saline or cisplatin (30 mg/kg), with or without palonosetron (a 5-HT3 receptor antagonist). *P < 0.05, LSD test, cisplatin or cisplatin + palonosetron compared with saline injection; aP < 0.05, LSD test, cisplatin vs. cisplatin + palonosetron.
To determine whether palonosetron might reduce basal levels of Fos expression in the PBN, CeA, and BNSTv we compared saline-saline- and palonosetron-saline-treated animals. There were no significant differences at 6 h between shrews treated with saline-saline and those treated with palonosetron-saline in PBN, CeA, or bed nucleus [all t(5 or 6) ≤ 1.5, all P ≥ 0.2; Fig. 8].
DISCUSSION
Cisplatin treatment induced Fos expression for at least 48 h in the DVC, PBN, and CeA of the house musk shrew, Suncus murinus, a species with a vomiting response. Furthermore, cisplatin-induced Fos expression in the DMN, NTS, and CeA at 6 h after injection was potently inhibited by pretreatment with the antiemetic palonosetron, indicating the involvement of 5-HT3 receptors. This is the first study using a vomiting species to show long-term activation of the hindbrain with cisplatin treatment, up to 48 h after treatment, and also in brain regions rostral to hindbrain emetic circuitry. These results indicate that cisplatin activates a neural system that includes several levels of the brain's neuroaxis and suggest potential brain areas that might play a role in the longer-term sickness produced by this chemotherapy agent.
Cisplatin-induced Fos expression indicates activation of emetic circuitry.
The antineoplastic agent cisplatin, a common chemotherapy agent, exerts its cytotoxic effects primarily through direct interference with DNA synthesis, replication, or repair (12). Cisplatin has many deleterious side effects, including the stimulation of nausea, vomiting, anorexia, and behaviors indicative of malaise (10, 14, 38, 39, 57). Potentially, any of these physiological effects might affect brain Fos expression. However, cisplatin's effects on Fos expression in specific brain areas known to play a role in emesis, and the inhibition of this response by an antiemetic agent, suggest that at least part of the Fos expression induced by cisplatin treatment in musk shrews is related to activation of emetic pathways.
Previous evidence indicates that cisplatin stimulates a vagal pathway to the hindbrain to produce emesis, and the present data showing cisplatin-induced Fos expression in the NTS and AP are consistent with this action. Although little neuroanatomic information is available for musk shrews, in the rat vagal afferents from the gastrointestinal tract project to the NTS and the AP (48). The initial stimulus for emesis induced by cisplatin appears to stem from the release of 5-HT from enterochromaffin cells, which activates vagal afferent fibers in the gut containing 5-HT3 receptors that project to the DVC (4, 45, 57). The essential neural circuitry for vomiting is contained in the hindbrain. A hindbrain that has been surgically disconnected from mid- and forebrain regions can still produce emetic-like responses, for example, in the cat (43). The DVC and regions of the reticular formation play an integral role in producing emesis (8, 19). In the present study, cisplatin treatment increased Fos expression in the NTS, AP, DMN, and reticular formation, which likely included activation of the nucleus ambiguous and C1/A1 regions. In our previous study (26) using the rat, a nonvomiting species, cisplatin injection induced Fos expression in the NTS and the AP but not in the DMN. DMN neurons make extensive projections to the upper gastrointestinal tract (e.g., in musk shrew, Ref. 74) and possibly initiate the giant retrograde contraction that moves intestinal contents back to the stomach before emesis, and the nucleus ambiguous likely participates in the esophageal shortening that occurs during vomiting (66). Therefore, cisplatin-induced Fos expression in the DMN might reflect the activation of motor outputs related to emesis.
Inhibition of cisplatin-induced hindbrain Fos expression by pretreatment with the antiemetic palonosetron, a 5-HT3 receptor antagonist, also indicates the activation of emetic circuits. In vomiting species, for example, humans, dogs, and ferrets, palonosetron is an effective antiemetic against chemotherapy-induced emesis, especially during the acute phase (15, 39). In the ferret, Fos expression in the NTS at ∼3 h after cisplatin injection was also reduced by pretreatment with granisetron, a 5-HT3 receptor antagonist (55). Cisplatin-induced Fos expression in the NTS of musk shrews, at least at 6 h after treatment, likely depends on vagal input, given that in the ferret vagotomy blocks cisplatin-induced Fos expression in the NTS (55). The NTS has a dense projection to the DMN in musk shrews and other animals (31, 56), and inhibition of DMN Fos expression by palonosetron is possibly due to suppression of activity in the NTS. Fos expression in the AP could reflect nonvagal action of cisplatin, because in the present study palonosetron did not greatly alter cisplatin-induced Fos expression in the AP, and because in the ferret vagotomy does not affect cisplatin-induced Fos expression in the AP (55). Cisplatin or humoral factors might activate the AP directly. For example, systemic injection of cisplatin increases plasma levels of substance P and 5-HT (23, 41), possibly derived from release of these factors from enteroendocrine cells of the gut. Direct application of 5-HT and substance P can activate neurons in the AP, and intracerebroventricular injection of cisplatin in the cat can produce emesis (11, 67).
Relationship between Fos expression and emesis 48 h after cisplatin treatment.
Although cisplatin treatment increased brain Fos expression for at least 48 h, Fos cell counts did not decline significantly over this duration. In a similar study in the rat, we also found that cisplatin treatment elevated Fos expression for at least 48 h after treatment (26). In addition, we have shown (13) that rats continue to ingest clay, a behavioral marker of sickness, for up to 2 wk after a single injection of cisplatin. The relationship in musk shrews between Fos expression and emesis produced by cisplatin treatment is not straightforward. In a study by Sam et al. (62) examining emetic episodes over 72 h, musk shrews vomited primarily within the first few hours of cisplatin treatment, followed by a prolonged (>24 h) period of little emesis, with a recurrence of emesis near 48 h after cisplatin injection. Long-term Fos expression in the emetic circuitry induced by cisplatin treatment might indicate a sustained level of activation of these nuclei that could contribute to nausea, malaise, and a reduced threshold for emesis, despite a period of little to no direct emetic behavior. In dogs, by 48 h after a bolus injection of cisplatin (3 mg/kg ip) only ∼40% of this chemical is recovered in urine, the primary method of elimination (35); therefore, the direct effects of cisplatin on physiology might persist for a significant period of time.
The present results also suggest that 5-HT3 receptors play little role in activation of Fos expression 48 h after cisplatin treatment, because palonosetron did not affect emesis at this time point. This is in agreement with results of another study on delayed emesis in musk shrews in which injection of granisetron, another 5-HT3 receptor antagonist, every 12 h also had no effects on emesis occurring from 24 to 72 h after cisplatin treatment (62). However, in the present study we administered palonosetron only once, 15 min before cisplatin injection, and it is unknown how long this agent remains active in musk shrews. An injection of palonosetron in humans has a half-life in plasma of 44–128 h (17).
The relative role of the gastrointestinal vagal afferent fibers and the area postrema in acute and delayed cisplatin-induced emesis is not well defined. Palonosetron significantly reduced cisplatin-induced Fos expression at 6 h in the NTS, and there was a trend for the AP (Fig. 7), but had no effect on 48 h Fos expression after cisplatin treatment. It is not clear where palonosetron might act to produce this effect because 5-HT3 receptors are present in the NTS, in the AP, and on vagal afferents (peripherally and centrally) (36, 54). 5-HT3 receptor antagonism produces a greater reduction in cisplatin-induced emesis compared with vagotomy (4, 62). Vagotomy in musk shrews appears to only delay cisplatin-induced emesis for the first few hours, with no actual reduction in the total amount of emesis over 72 h (62). There appear to be no reports of the effects of AP ablation on cisplatin-induced delayed emesis; however, this method of study could be difficult to interpret because vagal afferent fibers also terminate in the AP (48, 53).
Cisplatin-induced Fos expression in PBN and CeA.
Similar to the rat (65), in the musk shrew a substantial number of ascending fibers from the NTS and the AP terminate in the PBN of the midbrain (31). Although there are no reports of PBN outputs in musk shrews, the PBN of the rat has projections to the CeA and other forebrain areas (32) and forms a critical relay for viscerosensory pathways from hindbrain to forebrain (65). Lesions of the PBN in the rat demonstrate a critical role for this brain area in the formation of conditioned taste aversion produced by toxins, including lithium chloride and cyclophosphamide (46). Cyclophosphamide, like cisplatin, is a chemotherapy agent that appears to produce emesis by action on a 5-HT3 pathway from the gut (44). In the present study, cisplatin treatment in musk shrews induced Fos expression in the PBN at 24 and 48 h, indicating long-term activation of this nucleus.
The CeA is a highly differentiated nucleus that has afferent and efferent connections with cortices, PBN, hypothalamus, DVC, and other brain regions (3). In the present study, Fos expression in the CeA paralleled similar effects in the DVC after cisplatin treatment, and pretreatment with palonosetron inhibited Fos cell counts in both areas at 6 h after cisplatin treatment. Studies suggest that the CeA is an important integrator for feeding behavior, including conditioned flavor aversion (33), so is it difficult to say whether cisplatin-induced Fos expression in the CeA of musk shrews is a direct function of emetic behaviors or a generalized function of homeostatic and stress-related experience.
In summary, it is unknown why cisplatin induces Fos expression in the PBN and the CeA. Presumably, in areas rostral to the hindbrain emetic circuitry would not be needed for the emetic response to cisplatin treatment because cisplatin acts on a vagal pathway to produce emesis, at least acutely, and the caudal hindbrain is sufficient for generation of emetic output (44). Activation of the PBN and the CeA might be related to stress, disrupted gastrointestinal function, altered water intake, or decreased food intake that occurs with cisplatin treatment (10, 14, 71). Alternatively, these actions on the PBN and the CeA might represent a pathway for nausea, which likely involves a forebrain projection. Vomiting and nausea in humans can occur separately and likely engage neural systems that are at least partially separate (25, 64). Although it is very difficult to determine when laboratory animals are experiencing nausea, conditioned taste aversion has been used as a correlate of nausea in animal models (51). Importantly, lesions of the PBN disrupt conditioned taste aversion in rats (47).
It would be highly informative to assess Fos expression in multiple forebrain regions of musk shrews in order to determine cisplatin responsiveness over time. However, a detailed neuroanatomic atlas for S. murinus does not exist, making many regional and nucleus-specific analyses difficult. In the present study, we did observe Fos expression in the cortex and hypothalamus, but this labeling was not specific and also occurred in saline-treated control animals. As a treatment effect, amygdalar labeling was relatively easy to discern because it presented as a cluster of cells. We were also able to routinely see a clustering of Fos-expressing cells in the BNSTv. Although this part of the forebrain receives a dense projection from the NTS in the musk shrew (31), it did not show changes in Fos expression after cisplatin. This suggests that not all pathways from the NTS are activated by cisplatin treatment.
Perspectives and Significance
This is the first study using a vomiting species to show activation of the hindbrain and brain regions rostral to hindbrain emetic circuitry for up to 48 h after cisplatin treatment. This time course parallels the acute and delayed phases of nausea and emesis in cancer patients and emesis in musk shrews after cisplatin treatment (35, 62). Cisplatin treatment stimulated Fos expression in brain areas known to play a role in emesis, which was inhibited by the antiemetic agent palonosetron, suggesting that at least part of the Fos expression induced by cisplatin treatment is related to activation of emetic pathways. It is unknown why cisplatin stimulated Fos expression in the PBN and the CeA, because evidence indicates that cisplatin acts on a vagal pathway to induce emesis (4), at least acutely, and the hindbrain contains the circuitry to generate emesis (44). PBN and CeA activation by cisplatin might be related to nausea, stress, disrupted gastrointestinal function, or decreased food intake. The present findings suggest the existence of an integrated neural system that could be targeted to reduce chemotherapy-induced nausea, malaise, and vomiting during acute and delayed phases in cancer patients. There is a host of current and investigational antiemetic drugs that should be evaluated to suppress the activity of this neural system (24).
GRANTS
This work was supported by National Institutes of Health Grants DK-065971 and DC-000014.
Acknowledgments
The authors thank Matthew Rosazza and Liz Still for expert assistance in collecting these data. The authors also thank Dr. Emilie Rissman at the University of Virginia for providing the initial stock animals to create a musk shrew colony.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Aapro MS How do we manage patients with refractory or breakthrough emesis? Support Care Cancer 10: 106–109, 2002. [DOI] [PubMed] [Google Scholar]
- 2.Aapro MS, Grunberg SM, Manikhas GM, Olivares G, Suarez T, Tjulandin SA, Bertoli LF, Yunus F, Morrica B, Lordick F, Macciocchi A. A phase III, double-blind, randomized trial of palonosetron compared with ondansetron in preventing chemotherapy-induced nausea and vomiting following highly emetogenic chemotherapy. Ann Oncol 17: 1441–1449, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Akmaev IG, Kalimullina LB, Sharipova LA. The central nucleus of the amygdaloid body of the brain: cytoarchitectonics, neuronal organization, connections. Neurosci Behav Physiol 34: 603–610, 2004. [DOI] [PubMed] [Google Scholar]
- 4.Andrews PL, Davis CJ, Bingham S, Davidson HI, Hawthorn J, Maskell L. The abdominal visceral innervation and the emetic reflex: pathways, pharmacology, and plasticity. Can J Physiol Pharmacol 68: 325–345, 1990. [DOI] [PubMed] [Google Scholar]
- 5.Andrews PL, Horn CC. Signals for nausea and emesis: implications for models of upper gastrointestinal diseases. Auton Neurosci 125: 100–115, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andrews PL, Okada F, Woods AJ, Hagiwara H, Kakaimoto S, Toyoda M, Matsuki N. The emetic and anti-emetic effects of the capsaicin analogue resiniferatoxin in Suncus murinus, the house musk shrew. Br J Pharmacol 130: 1247–1254, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ariumi H, Saito R, Nago S, Hyakusoku M, Takano Y, Kamiya H. The role of tachykinin NK-1 receptors in the area postrema of ferrets in emesis. Neurosci Lett 286: 123–126, 2000. [DOI] [PubMed] [Google Scholar]
- 8.Beleslin DB, Krstic SK. Further studies on nicotine-induced emesis: nicotinic mediation in area postrema. Physiol Behav 39: 681–686, 1987. [DOI] [PubMed] [Google Scholar]
- 9.Bojkowska K, Hamczyk MM, Tsai HW, Riggan A, Rissman EF. Neuropeptide Y influences acute food intake and energy status affects NPY immunoreactivity in the female musk shrew (Suncus murinus). Horm Behav 53: 342–350, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cabezos PA, Vera G, Castillo M, Fernandez-Pujol R, Martin MI, Abalo R. Radiological study of gastrointestinal motor activity after acute cisplatin in the rat. Temporal relationship with pica. Auton Neurosci 141: 54–65, 2008. [DOI] [PubMed] [Google Scholar]
- 11.Carpenter DO, Briggs DB, Strominger N. Responses of neurons of canine area postrema to neurotransmitters and peptides. Cell Mol Neurobiol 3: 113–126, 1983. [DOI] [PubMed] [Google Scholar]
- 12.Cepeda V, Fuertes MA, Castilla J, Alonso C, Quevedo C, Perez JM. Biochemical mechanisms of cisplatin cytotoxicity. Anticancer Agents Med Chem 7: 3–18, 2007. [DOI] [PubMed] [Google Scholar]
- 13.Constenla M 5-HT3 receptor antagonists for prevention of late acute-onset emesis. Ann Pharmacother 38: 1683–1691, 2004. [DOI] [PubMed] [Google Scholar]
- 14.De Jonghe BC, Horn CC. Chemotherapy-induced pica and anorexia are reduced by common hepatic branch vagotomy in the rat. Am J Physiol Regul Integr Comp Physiol 294: R756–R765, 2008. [DOI] [PubMed] [Google Scholar]
- 15.Eglen RM, Lee CH, Smith WL, Johnson LG, Clark R, Whiting RL, Hegde SS. Pharmacological characterization of RS 25259-197, a novel and selective 5-HT3 receptor antagonist, in vivo. Br J Pharmacol 114: 860–866, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eisenberg P, Figueroa-Vadillo J, Zamora R, Charu V, Hajdenberg J, Cartmell A, Macciocchi A, Grunberg S. Improved prevention of moderately emetogenic chemotherapy-induced nausea and vomiting with palonosetron, a pharmacologically novel 5-HT3 receptor antagonist: results of a phase III, single-dose trial versus dolasetron. Cancer 98: 2473–2482, 2003. [DOI] [PubMed] [Google Scholar]
- 17.Eisenberg P, MacKintosh FR, Ritch P, Cornett PA, Macciocchi A. Efficacy, safety and pharmacokinetics of palonosetron in patients receiving highly emetogenic cisplatin-based chemotherapy: a dose-ranging clinical study. Ann Oncol 15: 330–337, 2004. [DOI] [PubMed] [Google Scholar]
- 18.Endo T, Minami M, Nakayasu M, Hirafuji M, Hamaue N, Omae N, Kang Y, Iwanaga T. Effects of granisetron and vagotomy on c-fos mRNA expression in the rat medulla oblongata as assessed by in situ hybridization. Biomed Res (Tokyo) 25: 229–235, 2004. [Google Scholar]
- 19.Fukuda H, Koga T, Furukawa N, Nakamura E, Hatano M, Yanagihara M. The site of the antiemetic action of NK1 receptor antagonists. In: Antiemetic Therapy, edited by Donnerer J. Basel: Karger, 2003, p. 33–77.
- 20.Fukui H, Yamamoto M. Methotrexate produces delayed emesis in dogs: a potential model of delayed emesis induced by chemotherapy. Eur J Pharmacol 372: 261–267, 1999. [DOI] [PubMed] [Google Scholar]
- 21.Gralla R, Lichinitser M, Van Der Vegt S, Sleeboom H, Mezger J, Peschel C, Tonini G, Labianca R, Macciocchi A, Aapro M. Palonosetron improves prevention of chemotherapy-induced nausea and vomiting following moderately emetogenic chemotherapy: results of a double-blind randomized phase III trial comparing single doses of palonosetron with ondansetron. Ann Oncol 14: 1570–1577, 2003. [DOI] [PubMed] [Google Scholar]
- 22.Hainsworth JD, Hesketh PJ. Single-dose ondansetron for the prevention of cisplatin-induced emesis: efficacy results. Semin Oncol 19: 14–19, 1992. [PubMed] [Google Scholar]
- 23.Higa GM, Auber ML, Altaha R, Piktel D, Kurian S, Hobbs G, Landreth K. 5-Hydroxyindoleacetic acid and substance P profiles in patients receiving emetogenic chemotherapy. J Oncol Pharm Pract 12: 201–209, 2006. [DOI] [PubMed] [Google Scholar]
- 24.Horn CC Is there a need to identify new anti-emetic drugs? Drug Discov Today Ther Strateg 4: 183–187, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Horn CC Why is the neurobiology of nausea and vomiting so important? Appetite 50: 430–434, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Horn CC, Ciucci M, Chaudhury A. Brain Fos expression during 48 h after cisplatin treatment: neural pathways for acute and delayed visceral sickness. Auton Neurosci 132: 44–51, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Horn CC, Richardson EJ, Andrews PL, Friedman MI. Differential effects on gastrointestinal and hepatic vagal afferent fibers in the rat by the anti-cancer agent cisplatin. Auton Neurosci 115: 74–81, 2004. [DOI] [PubMed] [Google Scholar]
- 28.Ito H, Nishibayashi M, Kawabata K, Maeda S, Seki M, Ebukuro S. Immunohistochemical demonstration of c-fos protein in neurons of the medulla oblongata of the musk shrew (Suncus murinus) after veratrine administration. Exp Anim 51: 19–25, 2002. [DOI] [PubMed] [Google Scholar]
- 29.Ito H, Nishibayashi M, Kawabata K, Maeda S, Seki M, Ebukuro S. Induction of Fos protein in neurons in the medulla oblongata after motion- and X-irradiation-induced emesis in musk shrews (Suncus murinus). Auton Neurosci 107: 1–8, 2003. [DOI] [PubMed] [Google Scholar]
- 30.Ito H, Nishibayashi M, Maeda S, Seki M, Ebukuro S. Emetic responses and neural activity in young musk shrews during the breast-feeding/weaning period: comparison between the high and low emetic response strains using a shaking stimulus. Exp Anim 54: 301–307, 2005. [DOI] [PubMed] [Google Scholar]
- 31.Ito H, Seki M. Ascending projections from the area postrema and the nucleus of the solitary tract of Suncus murinus: anterograde tracing study using Phaseolus vulgaris leucoagglutinin. Okajimas Folia Anat Jpn 75: 9–31, 1998. [DOI] [PubMed] [Google Scholar]
- 32.Jia HG, Rao ZR, Shi JW. An indirect projection from the nucleus of the solitary tract to the central nucleus of the amygdala via the parabrachial nucleus in the rat: a light and electron microscopic study. Brain Res 663: 181–190, 1994. [DOI] [PubMed] [Google Scholar]
- 33.Kinzig KP, D'Alessio DA, Seeley RJ. The diverse roles of specific GLP-1 receptors in the control food intake and the response to visceral illness. J Neurosci 22: 10470–10476, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kris MG, Gralla RJ, Clark RA, Tyson LB, Groshen S. Control of chemotherapy-induced diarrhea with the synthetic enkephalin BW942C: a randomized trial with placebo in patients receiving cisplatin. J Clin Oncol 6: 663–668, 1988. [DOI] [PubMed] [Google Scholar]
- 35.Kris MG, Hesketh PJ, Somerfield MR, Feyer P, Clark-Snow R, Koeller JM, Morrow GR, Chinnery LW, Chesney MJ, Gralla RJ, Grunberg SM. American Society of Clinical Oncology guideline for antiemetics in oncology: update 2006. J Clin Oncol 24: 2932–2947, 2006. [DOI] [PubMed] [Google Scholar]
- 36.Lagasse LD, Pretorius RG, Petrilli ES, Ford LC, Hoeschele J, Kean C. The metabolism of cis-dichlorodiammineplatinum (II): distribution, clearance, and toxicity. Am J Obstet Gynecol 139: 791–798, 1981. [DOI] [PubMed] [Google Scholar]
- 37.Leslie RA, Reynolds DJ, Andrews PL, Grahame-Smith DG, Davis CJ, Harvey JM. Evidence for presynaptic 5-hydroxytryptamine3 recognition sites on vagal afferent terminals in the brainstem of the ferret. Neuroscience 38: 667–673, 1990. [DOI] [PubMed] [Google Scholar]
- 38.Malik NM, Liu YL, Cole N, Sanger GJ, Andrews PL. Differential effects of dexamethasone, ondansetron and a tachykinin NK1 receptor antagonist (GR205171) on cisplatin-induced changes in behaviour, food intake, pica and gastric function in rats. Eur J Pharmacol 555: 164–173, 2007. [DOI] [PubMed] [Google Scholar]
- 39.Malik NM, Moore GB, Smith G, Liu YL, Sanger GJ, Andrews PL. Behavioural and hypothalamic molecular effects of the anti-cancer agent cisplatin in the rat: a model of chemotherapy-related malaise? Pharmacol Biochem Behav 83: 9–20, 2006. [DOI] [PubMed] [Google Scholar]
- 40.Massa E, Astara G, Madeddu C, Dessi M, Loi C, Lepori S, Mantovani G. Palonosetron plus dexamethasone effectively prevents acute and delayed chemotherapy-induced nausea and vomiting following highly or moderately emetogenic chemotherapy in pre-treated patients who have failed to respond to a previous antiemetic treatment: comparison between elderly and non-elderly patient response. Crit Rev Oncol Hematol (August 23, 2008); doi: 10.1016/j.critrevonc.2008.07.002. [DOI] [PubMed]
- 41.Matsuki N, Ueno S, Kaji T, Ishihara A, Wang CH, Saito H. Emesis induced by cancer chemotherapeutic agents in the Suncus murinus: a new experimental model. Jpn J Pharmacol 48: 303–306, 1988. [DOI] [PubMed] [Google Scholar]
- 42.Matsumoto S, Kawasaki Y, Mikami M, Nakamoto M, Tokuyasu H, Kometani Y, Chikumi H, Hitsuda Y, Matsumoto Y, Sasaki T. [Relationship between cancer chemotherapeutic drug-induced delayed emesis and plasma levels of substance P in two patients with small cell lung cancer]. Gan To Kagaku Ryoho 26: 535–538, 1999. [PubMed] [Google Scholar]
- 43.Milano S, Blower P, Romain D, Grelot L. The piglet as a suitable animal model for studying the delayed phase of cisplatin-induced emesis. J Pharmacol Exp Ther 274: 951–961, 1995. [PubMed] [Google Scholar]
- 44.Miller AD, Nonaka S, Jakus J. Brain areas essential or non-essential for emesis. Brain Res 647: 255–264, 1994. [DOI] [PubMed] [Google Scholar]
- 45.Miller AD, Ruggiero DA. Emetic reflex arc revealed by expression of the immediate-early gene c-fos in the cat. J Neurosci 14: 871–888, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Minami M, Endo T, Hirafuji M, Hamaue N, Liu Y, Hiroshige T, Nemoto M, Saito H, Yoshioka M. Pharmacological aspects of anticancer drug-induced emesis with emphasis on serotonin release and vagal nerve activity. Pharmacol Ther 99: 149–165, 2003. [DOI] [PubMed] [Google Scholar]
- 47.Mungarndee SS, Lundy RF Jr, Norgren R. Central gustatory lesions and learned taste aversions: unconditioned stimuli. Physiol Behav 87: 542–551, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nelson K, Walsh D, Sheehan F. Cancer and chemotherapy-related upper gastrointestinal symptoms: the role of abnormal gastric motor function and its evaluation in cancer patients. Support Care Cancer 10: 455–461, 2002. [DOI] [PubMed] [Google Scholar]
- 49.Norgren R, Smith GP. Central distribution of subdiaphragmatic vagal branches in the rat. J Comp Neurol 273: 207–223, 1988. [DOI] [PubMed] [Google Scholar]
- 50.Olson BR, Hoffman GE, Sved AF, Stricker EM, Verbalis JG. Cholecystokinin induces c-fos expression in hypothalamic oxytocinergic neurons projecting to the dorsal vagal complex. Brain Res 569: 238–248, 1992. [DOI] [PubMed] [Google Scholar]
- 51.Parker LA The role of nausea in taste avoidance learning in rats and shrews. Auton Neurosci 125: 34–41, 2006. [DOI] [PubMed] [Google Scholar]
- 52.Powley TL, Fox EA, Berthoud HR. Retrograde tracer technique for assessment of selective and total subdiaphragmatic vagotomies. Am J Physiol Regul Integr Comp Physiol 253: R361–R370, 1987. [DOI] [PubMed] [Google Scholar]
- 53.Ranson RN, Butler PJ, Taylor EW. The central localization of the vagus nerve in the ferret (Mustela putorius furo) and the mink (Mustela vison). J Auton Nerv Syst 43: 123–137, 1993. [DOI] [PubMed] [Google Scholar]
- 54.Raybould HE, Glatzle J, Robin C, Meyer JH, Phan T, Wong H, Sternini C. Expression of 5-HT3 receptors by extrinsic duodenal afferents contribute to intestinal inhibition of gastric emptying. Am J Physiol Gastrointest Liver Physiol 284: G367–G372, 2003. [DOI] [PubMed] [Google Scholar]
- 55.Reynolds DJ, Barber NA, Grahame-Smith DG, Leslie RA. Cisplatin-evoked induction of c-fos protein in the brainstem of the ferret: the effect of cervical vagotomy and the anti-emetic 5-HT3 receptor antagonist granisetron (BRL 43694). Brain Res 565: 231–236, 1991. [DOI] [PubMed] [Google Scholar]
- 56.Rogers RC, McCann MJ. Intramedullary connections of the gastric region in the solitary nucleus: a biocytin histochemical tracing study in the rat. J Auton Nerv Syst 42: 119–130, 1993. [DOI] [PubMed] [Google Scholar]
- 57.Rudd JA, Andrews PLR. Mechanisms of acute, delayed, and anticipatory emesis induced by anticancer therapies. In: Management of Nausea and Vomiting in Cancer and Cancer Treatment, edited by Hesketh PJ. Sudbury, MA: Jones & Bartlett, 2005, p. 15–65.
- 58.Rudd JA, Naylor RJ. Effects of 5-HT3 receptor antagonists on models of acute and delayed emesis induced by cisplatin in the ferret. Neuropharmacology 33: 1607–1608, 1994. [DOI] [PubMed] [Google Scholar]
- 59.Rudd JA, Ngan MP, Wai MK. Inhibition of emesis by tachykinin NK1 receptor antagonists in Suncus murinus (house musk shrew). Eur J Pharmacol 366: 243–252, 1999. [DOI] [PubMed] [Google Scholar]
- 60.Saeki M, Sakai M, Saito R, Kubota H, Ariumi H, Takano Y, Yamatodani A, Kamiya H. Effects of HSP-117, a novel tachykinin NK1-receptor antagonist, on cisplatin-induced pica as a new evaluation of delayed emesis in rats. Jpn J Pharmacol 86: 359–362, 2001. [DOI] [PubMed] [Google Scholar]
- 61.Sagar SM, Sharp FR, Curran T. Expression of c-fos protein in brain: metabolic mapping at the cellular level. Science 240: 1328–1331, 1988. [DOI] [PubMed] [Google Scholar]
- 62.Sam TS, Cheng JT, Johnston KD, Kan KK, Ngan MP, Rudd JA, Wai MK, Yeung JH. Action of 5-HT3 receptor antagonists and dexamethasone to modify cisplatin-induced emesis in Suncus murinus (house musk shrew). Eur J Pharmacol 472: 135–145, 2003. [DOI] [PubMed] [Google Scholar]
- 63.Sam TS, Ngan MP, Riendeau D, Robichaud A, Rudd JA. Action of cyclooxygenase inhibitors and a leukotriene biosynthesis inhibitor on cisplatin-induced acute and delayed emesis in the ferret. J Pharmacol Sci 103: 189–200, 2007. [DOI] [PubMed] [Google Scholar]
- 64.Sanger GJ, Andrews PL. Treatment of nausea and vomiting: gaps in our knowledge. Auton Neurosci 129: 3–16, 2006. [DOI] [PubMed] [Google Scholar]
- 65.Saper C Central autonomic nervous system. In: The Rat Nervous System, edited by Paxinos G. New York: Elsevier, 2004, p. 761–796.
- 66.Smith JE, Paton JF, Andrews PL. An arterially perfused decerebrate preparation of Suncus murinus (house musk shrew) for the study of emesis and swallowing. Exp Physiol 87: 563–574, 2002. [DOI] [PubMed] [Google Scholar]
- 67.Smith WL, Callaham EM, Alphin RS. The emetic activity of centrally administered cisplatin in cats and its antagonism by zacopride. J Pharm Pharmacol 40: 142–143, 1988. [DOI] [PubMed] [Google Scholar]
- 68.Takeda N, Hasegawa S, Morita M, Matsunaga T. Pica in rats is analogous to emesis: an animal model in emesis research. Pharmacol Biochem Behav 45: 817–821, 1993. [DOI] [PubMed] [Google Scholar]
- 69.Ueno S, Matsuki N, Saito H. Suncus murinus: a new experimental model in emesis research. Life Sci 41: 513–518, 1987. [DOI] [PubMed] [Google Scholar]
- 70.Van Sickle MD, Oland LD, Mackie K, Davison JS, Sharkey KA. Delta9-tetrahydrocannabinol selectively acts on CB1 receptors in specific regions of dorsal vagal complex to inhibit emesis in ferrets. Am J Physiol Gastrointest Liver Physiol 285: G566–G576, 2003. [DOI] [PubMed] [Google Scholar]
- 71.Vera G, Chiarlone A, Martin MI, Abalo R. Altered feeding behaviour induced by long-term cisplatin in rats. Auton Neurosci 126–127: 81–92, 2006. [DOI] [PubMed] [Google Scholar]
- 72.Warr DG, Grunberg SM, Gralla RJ, Hesketh PJ, Roila F, Wit R, Carides AD, Taylor A, Evans JK, Horgan KJ. The oral NK1 antagonist aprepitant for the prevention of acute and delayed chemotherapy-induced nausea and vomiting: pooled data from 2 randomised, double-blind, placebo controlled trials. Eur J Cancer 41: 1278–1285, 2005. [DOI] [PubMed] [Google Scholar]
- 73.Watson RE, Wiegand SJ, Clough RW, Hoffman GE. Use of cryoprotectant to maintain long-term peptide immunoreactivity and tissue morphology. Peptides 7: 155–159, 1986. [DOI] [PubMed] [Google Scholar]
- 74.Won MH, Matsuo K, Oh YS, Kitoh J. Brainstem topology of the vagal motoneurons projecting to the esophagus and stomach in the house musk shrew, Suncus murinus. J Auton Nerv Syst 68: 171–181, 1998. [DOI] [PubMed] [Google Scholar]