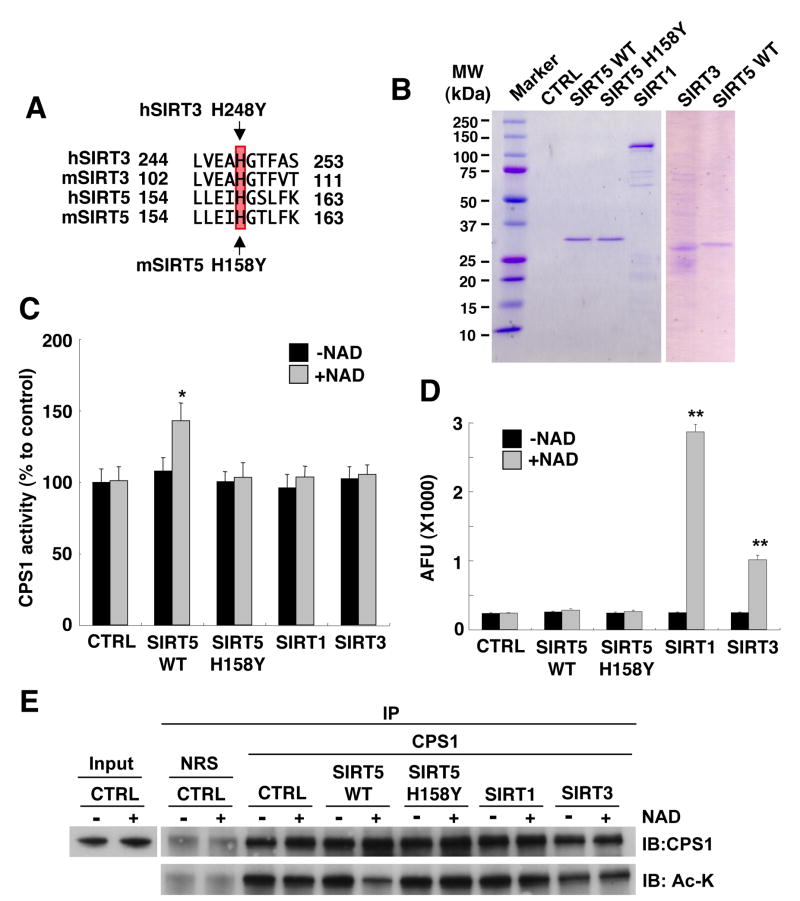
Figure 4. SIRT5 deacetylates and activates CPS1 in vitro.
(A) Comparison of partial amino acid sequence between human SIRT3 (hSIRT3), mouse SIRT3 (mSIRT3), human SIRT5 (hSIRT5), and mouse SIRT5 mSIRT5). H248 in human SIRT3 is also conserved in mouse and human SIRT5 as H158.
(B) Flag affinity purified mouse wild-type SIRT5-Flag (SIRT5 WT), mouse catalytic mutant SIRT5-Flag (SIRT5 H158Y), human SIRT3, human SIRT1 recombinant protein and Mock IP control were subjected to SDS-PAGE and stained with Coomasie Brilliant Blue.
(C) Flag affinity purified Flag affinity purified wild-type SIRT5, catalytic mutant SIRT5 H158Y, SIRT1, SIRT3 recombinant protein or Mock IP buffer (CTRL) were assayed for deacetylation using the p53 peptide-fluorescence based SIRT1 activity assay (BIO MOL). Error bars represent standard deviations from triplicate experiments. SIRT1 is shown to deacetylate this peptide, while SIRT5 does not.
(D) SIRT5 up-regulates CPS1 activity in vitro. Mitochondria matrix lysates from SIRT5 KO liver were incubated with Flag affinity purified wild-type SIRT5, catalytic mutant SIRT5 H158Y, SIRT1, SIRT3 recombinant protein or Mock IP buffer (CTRL) at 37 °C for 60 min in the presence or absence of NAD and subsequently subjected to the CPS1 activity assay. Error bars represent standard deviations from triplicate experiments. SIRT5 is shown to activate CPS1, while SIRT1 and SIRT3 does not.
(E) SIRT5 specifically deacetylates CPS1 in vitro. Mitochondria matrix lysates from SIRT5 KO liver were incubated with flag affinity purified wild-type SIRT5, catalytic mutant SIRT5 (H158Y), SIRT1, SIRT3 recombinant protein or mock IP buffer (CTRL) at 37 °C for 60 min in the presence or absence of NAD and subsequently subjected to immunoprecipitation with anti-CPS1 antibody or normal rabbit serum (NRS). Immuno-precipitates were analyzed by western blotting with anti-CPS1 and anti-pan acetylated lysine (Ac-K) antibodies.