Abstract
Reduced bone toughness, the energy absorption capacity of the tissue, has been consistently documented in vertebrae of animals treated with a wide range of bisphosphonate doses. Data regarding toughness changes in the rib are conflicting with one report showing no effect and another showing a significant reduction following treatment of beagle dogs with high doses of bisphosphonates. The goal of this study was to evaluate changes in bone toughness and various other tissue-level properties of the rib following three years of bisphosphonate treatment with doses at and above those used to treat osteoporosis. Skeletally mature intact beagle dogs were treated daily for three years with vehicle (VEH), alendronate 0.2 mg/kg/day (ALN0.2), or alendronate 1.0 mg/kg/day (ALN1.0). The lower ALN dose approximates, on a mg/kg basis, that used for treatment of postmenopausal osteoporosis with the higher dose being 5x higher. Ribs were assessed for biomechanical properties, bone turnover rate, microdamage, density, and geometry. Toughness was significantly lower with ALN1.0 (-33%), but not ALN0.2 (-19%), compared to VEH while neither ultimate stress nor modulus differed among groups. Bone density, geometry, and structural biomechanical properties were similar among the three groups. There was no significant difference in overall microdamage accumulation among groups. Intracortical bone formation rate was significantly lower than VEH in both ALN groups (-69 to -90%). These data show that while rib cortical bone experiences significant reductions in turnover following bisphosphonate treatment, it is only in animals treated with doses above those used to treat osteoporosis that toughness is significantly compromised.
Keywords: bisphosphonates, remodeling suppression, microcracks, biomechanical properties, cortical bone
Introduction
Reduced bone toughness, the energy absorption capacity of the tissue, has been consistently documented in the vertebrae of beagle dogs treated with bisphosphonates. Following one year of treatment at doses equivalent to or below those used to treat postmenopausal osteoporosis, bisphosphonates non-significantly reduced (-6 to -20%) vertebral bone toughness [1]; higher doses significantly reduced vertebral toughness (-21%) [2]. Bisphosphonate treatment for three years significantly reduced vertebral toughness compared to vehicle-treated controls at doses both equivalent to (-27%) and above (-30 to -40%) those used to treat patients with osteoporosis [3, 4].
Less is known regarding how bisphosphonate treatment affects toughness at other skeletal sites. Specifically, data regarding bisphosphonate-induced changes in bone toughness at the rib, a primarily cortical bone site that undergoes appreciable amounts of bone remodeling and is at risk for osteoporotic fracture, are conflicting. Significant reductions in toughness (-20%) of ribs from beagle dogs were shown after one year of alendronate treatment at doses exceeding those used clinically for postmenopausal osteoporosis [5]. In contrast, following three years of high dose incadronate treatment, no significant difference in rib toughness was shown between treated and non-treated animals [6]. Although these latter data were touted in an editorial to point to a new ‘vista’ with respect to the negative effects of bisphosphonates on material properties [7], in actuality the manner in which toughness was calculated in these two papers was significantly different. In three point bending tests, the load-deformation curve describes forces and moments that can be used to depict stresses and strains. This is achieved via transformation of the load-deformation curve into a stress-strain curve using standard engineering equations that incorporate calculations based on the dimensions of the sample rather than use direct values. This stress-strain curve can be broken down into pre-yield and post-yield portions (Figure 1). Pre-yield toughness represents the area under the stress/strain curve up to the point of yield, which is where permanent deformation of the bone has occurred while post-yield toughness represents the area under the curve between the yield point and fracture. In these rib bending tests, there exists a significant amount of displacement between the point of yield and the eventual fracture. Mashiba et al. defined toughness as the area under the stress-strain curve to fracture, therefore encompassing both pre-yield and post-yield regions [5]. Komatsubara et al. defined toughness as the area under the stress-strain curve to ultimate stress [7]. Given that yield and ultimate stress are nearly identical in these rib three point bending tests, the values reported by Komatsubara reflect mainly the pre-yield portion of the curve. Whether the different approaches to defining toughness fully explain these contrasting results is unclear.
Figure 1.
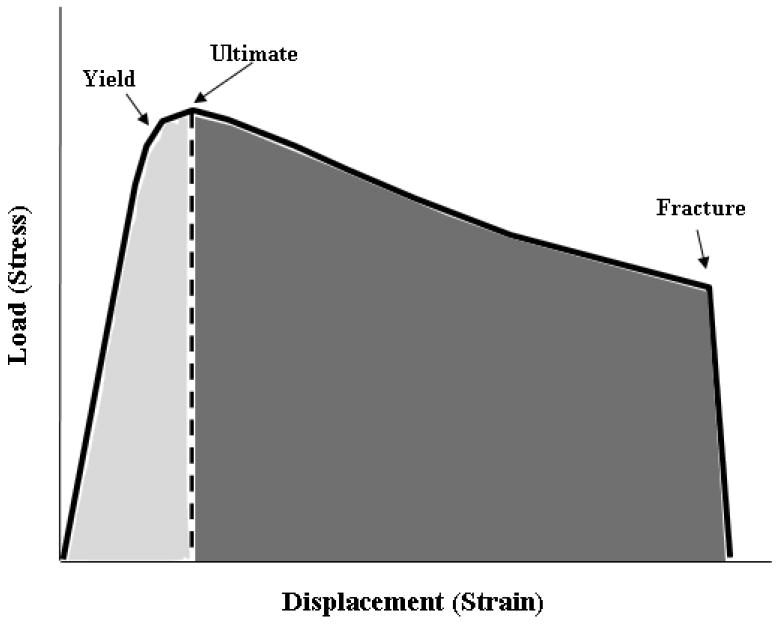
Schematic of mechanical test plot from three-point bending test of dog rib. Load versus deformation data from tests are converted to stress and strain, respectively, using standard equations (see methods for equations). In these tests, the yield stress and ultimate stress are nearly identical, while there is a considerable amount of displacement (strain) between these points and the point of fracture. In the current study, toughness (the area under the stress/strain curve) was quantified fracture (total area under the curve). We also separated the area under the curve into pre-yield toughness (area of the curve to ultimate stress, lightly shaded region) and post-yield toughness (area under the curve between ultimate stress and fracture, darkly shaded region). Previous analyses by Komatsubara [6] assessed toughness only to the point of ultimate stress.
The goal of this study was to evaluate differences in bone toughness, along with various other properties of the rib, following three years of daily alendronate treatment at doses equivalent to and above those used for treatment of post menopausal osteoporosis. We hypothesized that alendronate would reduce bone toughness at doses equal to and above those used clinically to treat osteoporosis, and that these changes would be associated with suppression of bone remodeling and accumulation of microdamage.
Materials and Methods
Experimental Design
Details of the experimental design have been previously published [3]. All procedures were approved by the Indiana University School of Medicine Animal Care and Use Committee. Female beagles (1-2 yrs old; n=36) were confirmed to be skeletally mature (closed proximal tibia and lumbar vertebra growth plates on x-ray) prior to the start of the study. Animals were treated daily for three years with oral doses of vehicle (VEH, 1 ml/kg/day saline) or alendronate (0.2, or 1.0 mg/kg/day, Merck and Co., Inc., Rahway, NJ). The 0.2 mg dose corresponds, on a mg/kg basis, to those used for treatment of post-menopausal osteoporosis while the 5x higher dose was chosen to match previous studies. Alendronate was mixed in saline as either a 0.05% solution (0.2 mg dose) or a 0.2% solution (1.0 mg dose) with a correction for the 16.4% moisture content. Appropriate amounts of the alendronate solution were supplemented with saline to reach a total volume of 10 mL for each daily dose. Dogs were administered alendronate, or a saline vehicle, with a syringe each morning after an overnight fast and at least 2 hours prior to feeding. Prior to necropsy, animals were injected with calcein (5 mg/kg as a 3% solution, IV) using a 2-12-2-5 labeling schedule to allow measurement of dynamic histomorphometry. Following three years of treatment, animals were euthanized by intravenous administration of sodium pentobarbital (0.22mg/kg Beuthanasia-D Special). The mid-portion of the ninth right rib was saved in 70% ethanol for assessment of microdamage while an adjacent portion was saved in 10% neutral buffered formalin for dynamic histomorphometry measurements. The left eleventh rib was wrapped in saline-soaked gauze and frozen (-20°C) for later measures of density, geometry, and mechanical testing.
Density and geometry
Volumetric bone density and geometry were quantified using a Norland Stratec XCT Research SA+ pQCT (Stratec Electronics). Prior to scanning, the point of greatest curvature on each rib (approximately mid-rib) was marked with a pencil and portions of the bone >20 mm from this point were removed (remaining specimen was 40 mm in total length). A single bone slice was imaged at the mid-point using a scanning resolution of 0.07 × 0.07 × 0.50 mm. Total bone mineral content (BMC, mg/mm), total volumetric bone mineral density (vBMD, mg/cm3), cross-sectional area (CSA, mm2), anterior-posterior diameter (APdia, mm) and cross-sectional moment of inertia (CSMI, mm4) were obtained using standard scanner software. CSMI values were calculated in the plane perpendicular to the axis of 3-point bending.
Histomorphometry (Dynamic and microdamage)
Ribs were processed separately for assessment of microdamage or fluorochrome labels. Specimens for microdamage analyses were stained en bloc with 1% basic fuchsin and then embedded undecalcified in methyl methacrylate [5]. Specimens for assessment of fluorochrome labels were embedded undecalcified in methyl methacrylate [8]. Two serial semi-thin sections (~80-100 μm) were cut from both stained and unstained ribs using a diamond wire saw (Histosaw; Delaware Diamond Knives). The unstained and bulk stained regions of assessment were ~ 5 mm apart. Microdamage and fluorochrome labels were assessed using a semiautomatic analysis system (Bioquant OSTEO 7.20.10, Bioquant Image Analysis Co.) attached to a microscope equipped with an ultraviolet light source (Nikon Optihot 2 microscope, Nikon). Measurements were carried out on one unstained (dynamic), and two bulk stained (microdamage) sections per animal. Analysis of two sections for microdamage assessment reduces the probability of not finding any cracks in a given specimen [9]. Dynamic histomorphometric measures included separate assessment of fluorochrome labels on the periosteal, endocortical/trabecular, and intracortical bone envelopes [8]. All variables were measured and calculated in accordance with ASBMR recommended standards [10]. Microdamage in the cortex of the rib (excluding trabecular bone) was assessed using UV fluorescence [1]. Measures included crack length (Cr.Le) and crack number (Cr.N) and calculations of crack density (Cr.Dn; Cr.N / bone area) and crack surface density (Cr.S.Dn; Cr.N * Cr.Le / bone area).
Biomechanical tests
Three-point bending was conducted in accordance with our previously described method [5] using a material testing machine (Alliance RT/5, MTS Systems Corp., Eden Prairie, MN, USA). After thawing to room temperature, specimens were placed on a three-point bending fixture (bottom support span = 25 mm) with the convex surface of the rib facing up. The upper support contact point was at the mid-point of the specimen, matching the site of pQCT analyses. Bones were soaked with PBS prior to starting the test. Specimens were loaded to failure at a displacement rate of 20 mm/min and load versus displacement data were collected at 10 Hz. Structural mechanical properties, ultimate load, stiffness, displacement, and energy absorption were determined from the load-deformation curves using standard definitions [5, 11]. Material-level properties, ultimate stress (σult), modulus (E), and toughness (u), were calculated by normalizing the structural parameters as follows:
where L represents the bottom support span of the testing fixture (25 mm) and APdia and CSMI are from pQCT scans. Displacement, energy absorption, and toughness parameters were calculated for the entire test (to fracture) as well as separately for the pre-yield and post-yield portions of the curve with the yield point defined using the 2% offset criterion [11]. Equations used to calculate material properties are identical to those used previously for testing dog ribs via three-point bending [5, 6].
Statistics
All statistical tests were performed using SAS software (SAS Institute, Inc.). Differences among the three treatment groups were evaluated using a one-way analysis of variance (ANOVA). When a significant overall F value (p ≤ 0.05) was present, differences between individual group means were tested using Fisher’s protected least-significant difference (PLSD) post-hoc test. For all tests, p ≤ 0.05 was considered significant. All data are presented as mean ± standard error.
Results
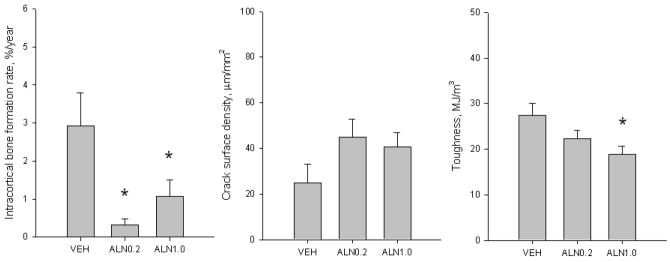
Toughness was significantly lower after three years of treatment with ALN1.0 (-33%), but not ALN0.2 (-19%), compared to VEH-treated animals (Figure 2). Specifically, there was a significant reduction in post-yield toughness with no difference among the three groups in pre-yield toughness (Table 1). Neither ultimate stress nor modulus differed among the three groups.
Figure 2.
Bone remodeling, microdamage accumulation, and toughness of rib cortical bone following three years of treatment with vehicle or alendronate (ALN). (A) Intracortical bone formation rate was significantly lower than vehicle in both ALN-treated groups while there was no difference between the two ALN doses (ANOVA p = 0.008). (B) Crack surface density, an index of microdamage accumulation representing the product of crack number and crack length normalized to bone area, was not significantly different from VEH with either dose of ALN (ANOVA p = 0.157). (C) Toughness, the energy absorption capacity to fracture, was significantly lower than VEH with ALN1.0 (-33%), but not with ALN0.2 (-19%) (ANOVA p = 0.023). Data expressed as mean ± SE. *p < 0.05 versus vehicle.
Table 1.
Rib density, geometry, and mechanical properties.
| Vehicle | Alendronate 0.2 mg/kg/day | Alendronate 1.0 mg/kg/day | P value | |
|---|---|---|---|---|
| vBMD, mg/cm3 | 1199 ± 10 | 1201 ± 11 | 1209 ± 9 | 0.768 |
| BMC, mg/mm | 8.48 ± 0.31 | 9.37 ± 0.41 | 9.58 ± 0.66 | 0.243 |
| CSMI, mm4 | 10.30 ± 0.93 | 11.66 ± 1.60 | 10.90 ± 1.67 | 0.799 |
| Ultimate load, N | 137 ± 8 | 142 ± 12 | 142 ± 14 | 0.950 |
| Stiffness, N/mm | 170 ± 9 | 184 ± 15 | 178 ± 21 | 0.801 |
| Pre-yield displacement, mm | 0.77 ± 0.01 | 0.78 ± 0.01 | 0.80 ± 0.02 | 0.343 |
| Post-yield displacement, mm | 4.73 ± 0.32 | 4.14 ± 0.26 | 3.30 ± 0.32 * | 0.008 |
| Energy to failure, mJ | 596 ± 55 | 568 ± 58 | 474 ± 54 | 0.283 |
| Ultimate stress, MPa | 168 ± 10 | 154 ± 8 | 159 ± 7.8 | 0.514 |
| Modulus, MPa | 5601 ± 318 | 5524 ± 320 | 5678 ± 407 | 0.953 |
| Pre-yield toughness, MJ/m3 | 3.2 ± 0.3 | 2.9 ± 0.2 | 2.9 ± 0.2 | 0.505 |
| Post-yield toughness, MJ/m3 | 24.2 ± 2.5 | 19.6 ± 1.6 | 16.0 ± 1.6 * | 0.019 |
Data are mean ± SE. p values refer to a one-way ANOVA among the three groups.
p < 0.05 versus Vehicle.
vBMD, volumetric bone mineral density; BMD, bone mineral content; CSMI, cross-sectional moment of inertia.
At the structural level, there was no significant difference among groups for ultimate load, stiffness, or energy to failure (Table 1). The ribs of animals treated with ALN1.0 had significantly reduced post-yield displacement (-20%) compared to VEH, consistent with the reductions in post-yield toughness, with no difference in pre-yield displacement (Table 1). There was no difference in bone mineral content, volumetric bone density, or cross-sectional moment of inertia among the three treatment groups (Table 1).
Bone formation rate (BFR/BS) on the endocortical/trabecular bone surface of alendronate-treated animals was significantly lower (-59% ALN0.2; -75% ALN1.0) compared to vehicle (Table 2). This reduction in BFR was the result of significant reductions in mineralizing surface (MS/BS) in both ALN groups with no significant difference in mineral apposition rate (MAR) among the groups. There was no difference in BFR on the endocortical/trabecular surface between the two doses of ALN. Suppression of BFR also occurred within the intracortical bone envelope, with both ALN0.2 (-90%) and ALN1.0 (-69%) having lower bone formation rates compared to VEH (Figure 2). Neither intracortical MAR nor labeled osteon number (a surrogate of MS/BS for the intracortical envelope) were significantly different among groups (Table 2), nor was there a difference between the two ALN doses for any intracortical turnover parameters. On the periosteal surface MS/BS was significantly higher in ribs of ALN0.2-treated animals compared to VEH and ALN1.0. Neither periosteal MAR nor BFR were different among the three groups.
Table 2.
Envelope-specific bone formation activity and intracortical microdamage.
| Vehicle | Alendronate 0.2 mg/kg/day | Alendronate 1.0 mg/kg/day | P value | |
|---|---|---|---|---|
| Periosteal | ||||
| MAR, μm/day | 1.09 ± 0.33 | 0.94 ± 0.17 | 0.41 ± 0.26 | 0.178 |
| MS/BS, % | 2.8 ± 0.9 | 5.8 ± 1.3 *† | 1.6 ± 0.6 | 0.012 |
| BFR, μm3/μm2/day | 6.6 ± 1.7 | 6.5 ± 1.3 | 2.3 ± 1.6 | 0.152 |
| Endocortical/Trabecular | ||||
| MAR, μm/day | 0.86 ± 0.07 | 0.83 ± 0.07 | 0.80 ± 0.13 | 0.904 |
| MS/BS, % | 13.8 ± 1.9 | 6.4 ± 1.5 * | 3.6 ± 0.9 * | <0.001 |
| BFR, μm3/μm2/day | 12.1 ± 2.0 | 6.7 ± 1.7 * | 4.4 ±1.6 * | 0.019 |
| Intracortical | ||||
| MAR, μm/day | 0.82 ± 0.12 | 0.31 ± 0.17 | 0.66 ± 0.16 | 0.067 |
| L.On.N, #/mm2 | 0.63 ± 0.12 | 0.24 ± 0.10 | 0.46 ± 0.18 | 0.148 |
| Crack number, # | 5.6 ± 1.6 | 9.4 ± 1.5 | 8.1 ± 0.9 | 0.171 |
| Mean crack length, μm | 58.9 ± 3.5 | 72.7 ± 2.2 * | 73.4 ± 3.4 * | 0.004 |
| Crack density, #/mm2 | 0.39 ± 0.11 | 0.60 ± 0.10 | 0.55 ± 0.08 | 0.305 |
Data are mean ± SE. p values refer to a one-way ANOVA among the three groups.
p < 0.05 versus Vehicle;
p < 0.05 versus ALN1.0. MAR, mineral apposition rate;
MS/BS, mineralizing surface per bone surface; BFR, bone formation rate; L.On.N, labeled osteon number.
There was no significant difference in microdamage accumulation (crack number, crack density, or crack surface density) within the cortical bone of the rib among the three treatment groups (Figure 2, Table 2). Mean crack length was significantly higher (+23%) in both ALN0.2 and ALN1.0 groups compared to VEH.
Discussion
The toughness of bone relates to its capacity to absorb energy and resist fracture. Data from biomechanical tests performed on the vertebrae of beagle dogs treated with bisphosphonates [1-4] consistently indicate that there is a reduction in toughness. However, reports of bisphosphonate-induced changes in rib toughness have thus far been discordant [5, 6]. Specifically, previous analyses of ribs from bisphosphonate-treated dogs showed significant reductions in toughness compared to vehicle-treated animals at one year using alendronate at doses 5x the clinical dose for osteoporosis, but not at three years using incadronate at 2.5x or 5x the clinical dose. The current study assessed changes in rib toughness, as well as other properties of the rib tissue, following three years of alendronate treatment at doses equivalent to or above those used for treatment of postmenopausal osteoporosis. We show that only animals treated with doses of alendronate 5x higher than those used for treatment of postmenopausal osteoporosis have significantly compromised toughness of the rib.
An important difference exists in the how the parameter of toughness was defined in previous studies that assessed rib biomechanical properties. One study revealed significant reductions in toughness following one year of alendronate treatment (using the same high dose used in the current study) and defined toughness as the area under the stress-strain curve to fracture [5]. Results of a subsequent study, in which the ribs from animals treated with high doses of incadronate (2.5x or 5x higher than the dose used for post-menopausal osteoporosis) showed no difference in toughness compared to controls, toughness was defined as the area under the stress-strain curve to ultimate stress [6]. As the yield and ultimate points are nearly identical in these three-point bending tests, energy absorption to ultimate stress reflects mainly the pre-yield portion of the curve. In these bending tests, the majority of energy absorption occurs in the post-yield region as is evident in the current experiment which demonstrated that >85% of toughness was contained in the post-yield region (Table 1). Based on this, it is likely that considering only the pre-yield region does not permit the detection of changes in rib toughness with bisphosphonate treatment. We demonstrate that there were no significant treatment-related differences in pre-yield toughness of the ribs and that the significant reduction in overall toughness (the energy absorption to fracture) can be attributable to reductions in post-yield toughness. While the clinical relevance of changes in pre- and post-yield toughness has not been clearly defined, it is generally considered that reductions in post-yield toughness compromise the ability to withstand an impact without fracture [11, 12].
While toughness of the rib was significantly lower than vehicle-treated controls in animals treated with the higher dose of alendronate (-33%), it was not significantly different at doses equivalent, on a mg/kg basis, to those used for treatment of postmenopausal osteoporosis (-19% compared to vehicle). It is worth noting that while the higher dose of alendronate used in this study approximates that used for Paget’s disease (our dose is about 25% higher on a mg/kg basis), our dosing schedule (daily for three years) exceeds that used clinically. While our data suggests a potential dose-dependent reduction in toughness, there was no significant difference when comparing the two alendronate doses. Despite reductions in toughness with the higher dose of alendronate, there was no significant effect on rib ultimate stress or modulus, pre-yield measures of material strength and stiffness, respectively. This is consistent with previous reports [5, 6] and suggests a specific effect of bisphosphonates on post-yield properties. Furthermore, there was no significant effect of alendronate treatment on the extrinsic properties of strength, stiffness, or energy absorption. This indicates that while the higher dose of alendronate does not affect the biomechanical integrity of the rib as a structural unit, probably because of the 13% greater BMC and 6% larger CSMI, the biomechanical properties of the material are likely compromised. This is similar to the changes in structural versus material properties that occur in vertebrae as a result of bisphosphonate treatment [3, 13].
Consistent with previous reports, we found significantly lower levels of bone remodeling on the endocortical/trabecular surfaces of ribs from animals treated with alendronate compared to vehicle. After one year of alendronate treatment, the bone formation rate on this bone surface was 75% lower than vehicle for the lower alendronate dose (0.2 mg) [8] and 78% lower with the higher alendronate dose (1.0 mg) [5]. Interestingly, the absolute level of bone formation on this surface after three years (6.7 μm3/μm2/day for ALN0.2 and 4.4 μm3/μm2/day for ALN1.0) were nearly identical to the levels after one year (6.5 μm3/μm2/day for ALN0.2 and 3.2 μm3/μm2/day for ALN1.0) [5, 8]. This suggests a lower limit of turnover suppression on the endocortical/trabecular bone surface of the rib which is maximal within the first year of treatment. Data from iliac crest biopsies of humans treated with bisphosphonates support the idea of a lower limit, with similar levels of turnover suppression on trabecular/endocortical surfaces in patients treated for 22 and 34 months with ibandronate [14]. Systemic lower limits of turnover suppression have also been noted in numerous studies of serum/urine biomarkers from patients treated with bisphosphonates [15].
The dynamics of remodeling suppression with bisphosphonates differ within the intracortical envelope of the rib. One year of treatment at the lower dose of alendronate (ALN0.2) did not significantly reduce intracortical turnover of the rib [8], while three years reduced the rate by 90% (Figure 2). Following one year of high-dose alendronate treatment (ALN1.0), intracortical turnover was reduced by 72% compared to controls [5], while the current study shows a 69% lower turnover after three years compared to controls. These data suggest that changes within the intracortical envelope of the rib are dependent on treatment duration and are slower to manifest with doses that are equivalent to those given clinically for treatment of postmenopausal osteoporosis compared to higher doses.
The overall level of microdamage was not significantly different among the three treatment groups; although significantly higher levels of microdamage in the rib were observed following one year of treatment with the higher dose of alendronate [5]. We interpret these data as suggesting the accumulation of damage is greatest during the early periods of bisphosphonate treatment, and subsequently achieves a new balance at the lower remodeling rate by three years. This is consistent with the kinetics of microdamage accumulation in the vertebrae that have been described previously in these same animals [3]. Also consistent with the results from the vertebra of these same animals is the finding that changes in toughness associated with bisphosphonate treatment are not congruent with changes in microdamage accumulation. There was a significant reduction in toughness (-33%) despite the absence of any significant difference in microdamage between animals treated with vehicle and those treated with the higher dose of alendronate. While it remains possible that microdamage may contribute to a portion of the changes in toughness with bisphosphonate treatment, there is a clear need to begin exploring how other changes in the tissue (e.g. changes to both the inorganic and the organic fractions of bone) contribute to the reduction in bone toughness following bisphosphonate treatment.
In conclusion these data show that while cortical bone sites undergo significant reductions in turnover in response to bisphosphonate treatment, it is only animals treated with doses that exceed those used for treatment of post menopausal osteoporosis in which toughness is significantly compromised. Furthermore, these results suggest that microdamage is unlikely a primary factor contributing to reductions in bone toughness with bisphosphonate treatment.
Acknowledgments
The authors thank Dr. Keith Condon, Diana Jacob, and Lauren Waugh for histological preparation. This work was supported by NIH Grants AR047838 and AR007581. Merck and Co. kindly provided the alendronate. This investigation utilized an animal facility constructed with support from Research Facilities Improvement Program Grant Number C06 RR10601-01 from the National Center for Research Resources, National Institutes of Health.
References
- 1.Allen MR, Iwata K, Phipps R, Burr DB. Alterations in canine vertebral bone turnover, microdamage accumulation, and biomechanical properties following 1-year treatment with clinical treatment doses of risedronate or alendronate. Bone. 2006;39:872–879. doi: 10.1016/j.bone.2006.04.028. [DOI] [PubMed] [Google Scholar]
- 2.Mashiba T, Turner CH, Hirano T, Forwood MR, Johnston CC, Burr DB. Effects of suppressed bone turnover by bisphosphonates on microdamage accumulation and biomechanical properties in clinically relevant skeletal sites in beagles. Bone. 2001;28:524–531. doi: 10.1016/s8756-3282(01)00414-8. [DOI] [PubMed] [Google Scholar]
- 3.Allen MR, Burr DB. Three years of alendronate treatment results in similar levels of vertebral microdamage as after one year of treatment. J Bone Miner Res. 2007;22:1759–1765. doi: 10.1359/jbmr.070720. [DOI] [PubMed] [Google Scholar]
- 4.Komatsubara S, Mori S, Mashiba T, Ito M, Li J, Kaji Y, Akiyama T, Miyamoto K, Cao Y, Kawanishi J, Norimatsu H. Long-term treatment of incadronate disodium accumulates microdamage but improves the trabecular bone microarchitecture in dog vertebra. J Bone Miner Res. 2003;18:512–520. doi: 10.1359/jbmr.2003.18.3.512. [DOI] [PubMed] [Google Scholar]
- 5.Mashiba T, Hirano T, Turner CH, Forwood MR, Johnston CC, Burr DB. Suppressed bone turnover by bisphosphonates increases microdamage accumulation and reduces some biomechanical properties in dog rib. J Bone Miner Res. 2000;15:613–620. doi: 10.1359/jbmr.2000.15.4.613. [DOI] [PubMed] [Google Scholar]
- 6.Komatsubara S, Mori S, Mashiba T, Li J, Nonaka K, Kaji Y, Akiyama T, Miyamoto K, Cao Y, Kawanishi J, Norimatsu H. Suppressed bone turnover by long-term bisphosphonate treatment accumulates microdamage but maintains intrinsic material properties in cortical bone of dog rib. J Bone Miner Res. 2004;19:999–1005. doi: 10.1359/JBMR.040126. [DOI] [PubMed] [Google Scholar]
- 7.Komatsubara S, Mori S, Mashiba T, Li J, Nonaka K, Kaji Y, Akiyama T, Miyamoto K, Cao Y, Kawanishi J, Norimatsu H. Suppressed bone turnover by long-term bisphosphonate treatment accumulates microdamage but maintains intrinsic material properties in cortical bone of dog rib (JBMR Anniversay Classic reprint) J Bone Miner Res. 2005;20:2066. doi: 10.1359/JBMR.040126. [DOI] [PubMed] [Google Scholar]
- 8.Allen MR, Follet H, Khurana M, Burr DB. Anti-remodeling agents influence osteoblast activity differently in modeling- and remodeling-associated bone formation. Calcified Tissue International. 2006;79:255–261. doi: 10.1007/s00223-006-0031-5. [DOI] [PubMed] [Google Scholar]
- 9.Martin RB, Yeh OC, Fyhrie DP. On sampling bones for microcracks. Bone. 2007;40:1159–1165. doi: 10.1016/j.bone.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 10.Parfitt A, Drezner M, Glorieux F, Kanis J, Malluche H, Meunier P, Ott S, Recker R. Bone histomorphometry: Standardization of nomenclature, symbols, and units. Journal of Bone and Mineral Research. 1987;2:595–610. doi: 10.1002/jbmr.5650020617. [DOI] [PubMed] [Google Scholar]
- 11.Turner CH, Burr DB. Basic biomechanical measurements of bone: a tutorial. Bone. 1993;14:595–608. doi: 10.1016/8756-3282(93)90081-k. [DOI] [PubMed] [Google Scholar]
- 12.Currey J. Incompatible mechanical properties in compact bone. J Theor Biol. 2004;231:569–580. doi: 10.1016/j.jtbi.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 13.Allen MR, Burr DB. Changes in vertebral strength-density and energy absorption-density relationships following bisphosphonate treatment in beagle dogs. Osteoporos Int. 2008;19:95–99. doi: 10.1007/s00198-007-0451-8. [DOI] [PubMed] [Google Scholar]
- 14.Recker RR, Weinstein RS, Chesnut CH, 3rd, Schimmer RC, Mahoney P, Hughes C, Bonvoisin B, Meunier PJ. Histomorphometric evaluation of daily and intermittent oral ibandronate in women with postmenopausal osteoporosis: results from the BONE study. Osteoporos Int. 2004;15:231–237. doi: 10.1007/s00198-003-1530-0. [DOI] [PubMed] [Google Scholar]
- 15.Bone HG, Hosking D, Devogelaer J-P, Tucci JR, Emkey RD, Tonino RP, Rodriguez-Portales JA, Downs RW, Gupta J, Santora AC, Liberman UA Alendronate Phase III Osteoporosis Treatment Study Group. Ten Years’ Experience with Alendronate for Osteoporosis in Postmenopausal Women. N Engl J Med. 2004;350:1189–1199. doi: 10.1056/NEJMoa030897. [DOI] [PubMed] [Google Scholar]