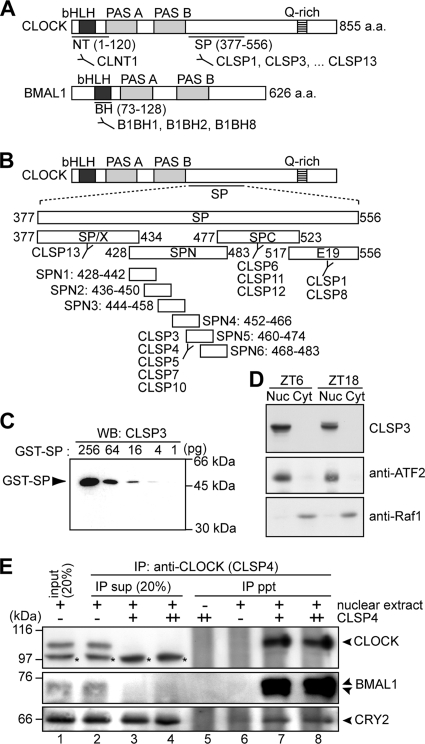
FIG. 1.
Characterization of anti-CLOCK and anti-BMAL1 MAbs. (A) Domain structures of CLOCK and BMAL1. Antigenic regions for anti-CLOCK and anti-BMAL1 MAbs are indicated. a.a., amino acids. (B) To localize epitopes of anti-SP antibodies, four partial peptides of SP, SP/X (Ser377-Ser434), SPN (Pro428-Glu483), SPC (Arg477-Met523), and E19 (Ser517-Glu556), were expressed as GST fusion proteins, which were recognized by CLSP13 (GST-SP/X); by CLSP3, -4, -5, -7, and -10 (GST-SPN); by CLSP6, -11, and -12 (GST-SPC); and by CLSP1 and -8 (GST-E19). For detailed determination of the epitopes, six shorter peptides of SPN, SPN1 (Pro428-His442), SPN2 (Ser436-Ser450), SPN3 (Ala444-Asp458), SPN4 (Pro452-His466), SPN5 (Pro460-Thr474), and SPN6 (Pro468-Glu483), were expressed as GST fusion proteins, among which GST-SPN5 was recognized by all of the SPN-immunoreactive antibodies (CLSP3, -4, -5, -7, and -10). (C) The immunizing peptide SP (Ser377-Glu556) was expressed as a GST fusion protein (GST-SP) and subjected to immunoblot analysis with CLSP3 anti-CLOCK MAb. The amounts of the fusion protein are indicated. WB, Western blotting. (D) The nuclear extract (Nuc) (40 μg of proteins) and the cytoplasmic fraction (Cyt) (40 μg of proteins) were prepared from the mouse liver at ZT6 and ZT18 and subjected to immunoblot analysis with CLSP3 anti-CLOCK MAb (top). Immunoblot analysis with anti-ATF2 antibody (middle) and anti-Raf1 antibody (bottom) supported the separation of nuclear and cytoplasmic proteins. (E) The nuclear extract (100 μg of proteins) prepared at ZT18 was immunoprecipitated with increasing amounts, 0.5 μg (+) and 1.0 μg (++), of CLSP4 anti-CLOCK MAb. The immunoprecipitates (ppt) and 20% of the remaining supernatants (sup) were subjected to immunoblot analysis with anti-CLOCK polyclonal antibody (Affinity BioReagents) (top), B1BH2 anti-BMAL1 MAb (middle), and anti-CRY2 polyclonal antibody (a kind gift of T. Todo) (bottom). The anti-CLOCK polyclonal antibody detected additional protein bands (shown by asterisks) that were invisible in the IP pellet in the presence of CLSP4 MAb, indicating that CLOCK was specifically immunoprecipitated by the antibody.