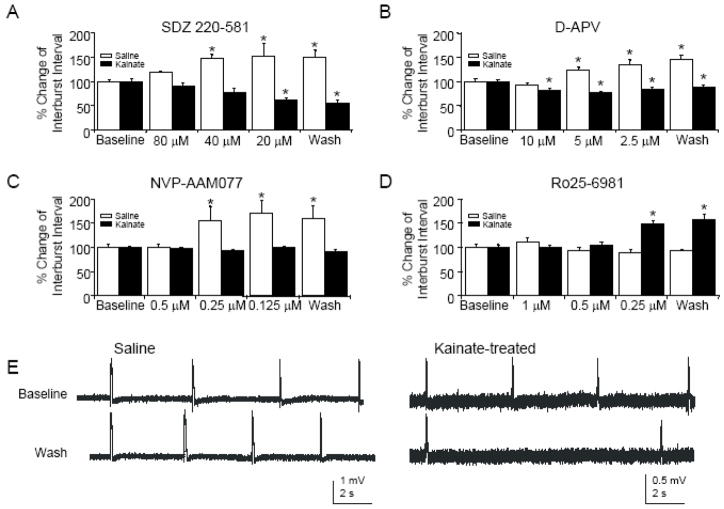
Figure 5.
In hippocampal slices prepared from chronically epileptic animals, only an NR2B-selective NMDAR antagonist induces activity-dependent depotentiation. A: Following bath application of SDZ, there are significant increases in intervals between spontaneous CA3 population bursts induced by pharmacological disinhibition in controls (n=6, ANOVA, P<0.01) but significant decreases in interburst intervals in kainite-treated rats (n=5, ANOVA, P<0.001). B: Similarly, following D-APV application, there are significant increases in CA3 interburst intervals in controls (n=7, ANOVA, P<0.01) and significant decreases in CA3 interburst intervals in kainite-treated rats (n=8, ANOVA, P<0.01). C: Partial blockade of NMDAR using an NR2A-subunit-selective antagonist significantly increases interburst intervals in saline-treated rats (n=6, ANOVA, P<0.01), while no change in interburst interval is observed in rats with kainite-induced epilepsy (n=6, ANOVA, P=0.18). D: Partial blockade of NMDAR using an NR2B-subunit-selective antagonist does not change interburst intervals in saline-treated rats (n=5, ANOVA, P=0.12). However, the same application produces significant increases in interburst intervals in chronically epileptic rats (n=5, ANOVA, P<0.001). E: Sample CA3 bursts from hippocampal slices prepared from saline- (left) and kainite- (right) treated rats before and after application of decreasing concentrations of the NR2B-selective NMDAR antagonist Ro25-6981. Asterisks represent significance compared to baseline.