Abstract
Purpose
The optimal chemotherapeutic strategy for gastric cancer patients has not been determined, especially with respect to stage and the curability of gastric cancer. The aim of this study was to evaluate the results of adjuvant chemotherapy on stage IV (T4N1-3M0 and T1-3N3M0) gastric cancer after curative gastrectomy between a chemotherapy (CTX) group and non-chemotherapy (non-CTX) group.
Materials and Methods
Among 1,760 patients who underwent gastric surgery by 1 surgeon in a single institution, 162 stage IV gastric cancer patients with curative gastrectomy were analyzed retrospectively, excluding patients with TanyNanyM1. One hundred twenty-five patients who received different chemotherapeutic regimens were compared to 37 patients who did not receive chemotherapy for reasons of old age or according to their expressed desire.
Results
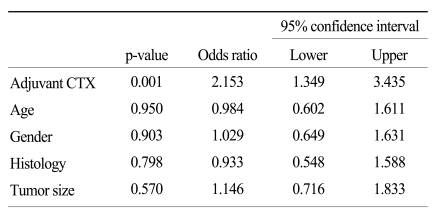
The clinicopathologic factors which showed a clinically significant difference between the two groups were age and histology, which were not associated with patient survival. The CTX group was younger, and had a larger proportion of undifferentiated gastric cancers than the non-CTX group. The mode of treatment failure revealed no significant difference between the CTX and non-CTX groups. The 1, 3, and 5-year disease-free survival and the 1, 3, and 5-year disease-specific survival of the CTX group were 63.9%, 38.4%, and 32.0%, and 85.4%, 52.3%, and 39.6%, respectively, which were more favorable than the non-CTX group (p=0.015 and p=0.001, respectively). Postoperative adjuvant CTX was an independent risk factor for disease-specific survival of stage IV (T4N1-3M0 and T1-3N3M0) gastric cancer patients after curative gastrectomy by multivariate analysis (odds ratio=2.153; 95% confidence interval=1.349-3.435; p=0.001).
Conclusions
Adjuvant CTX may be associated with survival benefit for younger patients with stage IV (T4N1-3M0 and T1-3N3M0) gastric cancer with undifferentiated histology after curative gastrectomy. A randomized controlled trial to reveal the effect of stage-specific adjuvant chemotherapy should be conducted.
Keywords: Adjuvant chemotherapy, Stage IV gastric cancer, Curative gastrectomy, survival
Introduction
Surgery remains the only curative treatment option in gastric cancer; however, the recurrence rate is still high, despite complete resection of primary tumor. The 5-year survival rate for all patients is not satisfactory and ranges from 10% to 53% (1). Chemotherapy (CTX) with various regimens have been administered to increase the survival rate. Over the past decades, many institutions have carried out clinical trials to achieve this with adjuvant therapy of gastric cancer and, in particular, to determine whether CTX after curative resection may improve survival compared to surgery alone. The first meta-analysis on adjuvant CTX after curative gastrectomy was published by Hermans et al. (2). In this report, postoperative CTX did not improve survival of gastric cancer with curative resection, and thus should not be considered as standard treatment. The other meta-analyses show that adjuvant CTX resulted in a significant survival advantage (3-6). The controversy remains unresolved, including the optimal chemotherapeutic regimen, the efficacy of new chemotherapeutic agents, and the method by which to compensate for toxicities in adjuvant chemotherapy. The effect of CTX according to the stage of gastric cancer has not been determined and remains unresolved. The aim of the present study was to retrospectively evaluate whether adjuvant CTX improves survival of stage IV (T4N1-3M0 and T1-3N3M0) gastric cancer patients who have undergone curative gastrectomy.
Materials and Methods
We retrospectively reviewed 162 stage IV gastric cancer patients who underwent curative gastrectomy, consisting of an absence of distant metastases, negative resection margins, no residual tumors, and > D2 lymphadenectomy by 1 surgeon in our hospital between June 1992 and December 2006. Stage IV gastric cancer with curability was defined based on the American Joint Commission on Cancer (AJCC, 6th edition), as T4N1-3M0 and T1-3N3M0 (7).
The 162 patients who underwent gastrectomy with curative intent were classified into the following 2 groups: one group received adjuvant CTX and the other group did not receive CTX (non-CTX). The CTX was started between 2 and 6 weeks postoperatively after patients reached ECOG performance status 0~2 (8). The chemotherapeutic regimens based on cisplatin included 5-FU, epirubicin, cisplatin, and methotrexate (FEPMTX; n=57), taxotere and cisplatin (TP; n=8), 5-FU and cisplatin (FP; n=27), S-1 and cisplatin (S-1/CDDP; n=31), and irinotecan and cisplatin (CPT11; n=2). The CTX group was designated if the patients received more than one cycle. The patients >75 years of age or who declined to accept CTX were designated as the non-CTX group. One hundred twenty-five patients received CTX, and 37 patients did not receive CTX.
1. Follow-up evaluation
The follow-up evaluation of patients after gastrectomy were performed every 3 months for the first 2 years, and then every 6 months for at least 5 years. Follow-up evaluations consisted of computed tomography of the abdomen, esophagogastroduodenoscopy, chest radiography, and barium enema. Whenever patients had clinical symptoms that suggested recurrence of disease, additional diagnostic tools, including bone scintigraphy, cytology, biopsy, and positron emission tomography were used to detect the presence of recurrence. The last follow-up of the patients continued until May 2008. Twenty patients were lost during the follow-up period (20/162 [12.4%]). The median follow-up duration for the 162 patients was 20.1 months (range, 2~164 months).
2. Statistical analysis
The statistical analysis was carried out using the statistical software, Statistical Package for the Social Sciences (SPSS), version 12.0 for Windows (SPSS, Inc., Chicago, IL). Student's t-test was used for comparison of means. Continuous variables were transformed to dichotomous variables in survival analysis. Disease-specific survival was calculated using the Kaplan-Meier method, and the difference between the survival curves was analyzed by a log-rank test. The patients that died of disease unrelated to gastric cancer were excluded from the analysis. Disease-free survival was calculated by the life table method, and the differences in survival were analyzed by the generalized Wilcoxon test. A p-value<0.05 was considered statistically significant.
Results
1. Clinicopathologic characteristics of stage IV (T4N1-3M0 and T1-3N3M0) gastric cancer patients
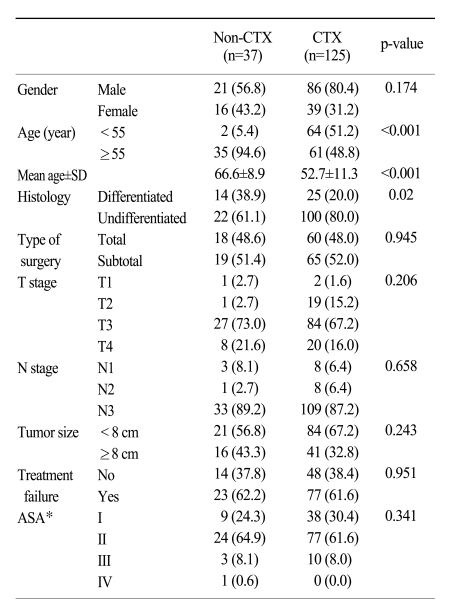
As shown in Table 1, the clincopathologic characteristics of the stage IV gastric cancer patients between the non-CTX and CTX groups were similar to each other, with the exception of age and histology. The patients <55 years of age received more frequent adjuvant chemotherapy compared to those >55 years of age (51.2% versus 5.4%, p<0.001). The mean age of the non-CTX and CTX groups was 66.6 and 52.7 years, respectively (p<0.001). The undifferentiated type of gastric cancer in the CTX group was significantly more frequent than in the non-CTX group (80.0% versus 61.1%, p=0.02). The depth of invasion of the tumor and lymph node metastasis, which are the most important factors affecting patients survival, were not significantly different between the two groups (9,10). The mean tumor size of all the patients was 7.8 cm (range, 3~20 cm).
Table 1.
Characteristics of stage IV gastric cancer patients with curative gastrectomy based on administration of chemotherapy
Number in parenthesis refer to percent.
*American society of anesthesiologists physical status classification.
2. Treatment failure
During the follow-up period after curative gastrectomy, 100 (61.7%) of 162 stage IV gastric cancer patients had recurrences of gastric cancer. The mean time to recurrence was 17 months (range, 1~120 months). The most frequent mode of treatment failure in stage IV gastric cancer was peritoneal dissemination, followed by distant metastasis, locoregional metastasis, and combined metastases (Table 2). The sites of distant metastasis included the liver (8), lung (4), brain (6), bone (2), skin (4), and Virchow's lymph node (1). The differences in mode of treatment failure in gastric cancer patients with CTX and non-CTX groups revealed no statistical significance (p=0.332). The palliative chemotherapy was conducted in 30 of 100 recurrent gastric cancer patients. All 30 patients had received adjuvant chemotherapy after curative gastrectomy.
Table 2.
Site of metastases in stage IV gastric cancer patients after curative gastrectomy based on administration of chemotherapy
Number in parenthesis refer to percent.
During the follow-up period, 103 patients died of various causes, including 89 gastric cancers, 6 unknown causes, 6 medical diseases, 1 non-gastric cancer, and 1 automobile accident.
3. Survival outcomes
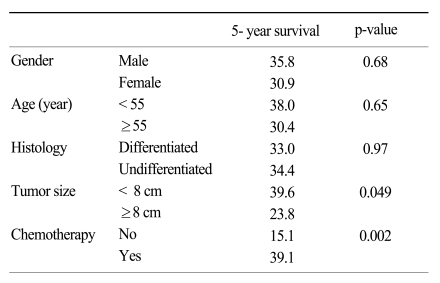
The risk factors related to disease-specific survival were tumor size and adjuvant CTX based on univariate analysis (p=0.049 and 0.002, respectively; Table 3). The age and histology showing a difference between the non-CTX and CTX group based on univariate analysis (Table 1) were not considered significant risk factors for disease-specific survival by using the Kaplan-Meier method.
Table 3.
The risk factors affecting 5-year survival rate in stage IV gastric cancer patients with curative gastrectomy using univariate analysis
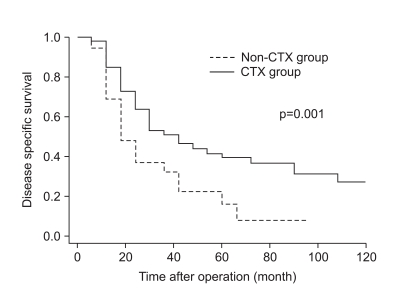
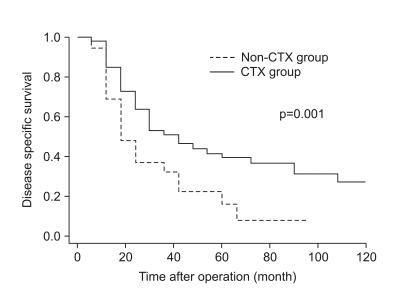
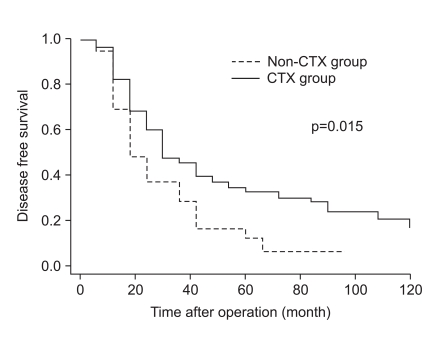
As shown in Fig. 1, the disease-specific survival of 1, 3, and 5 years of the 2 groups analyzed by the Kaplan-Meier method showed a significant difference (85.4%, 52.3%, and 39.6% in CTX vs. 62.5%, 30.6%, and 14.5% in non-CTX groups, respectively; Fig. 1; p=0.001). In analyzing 1, 3, and 5 year disease-free survival using the life table method, the CTX group had more a favorable result compared to the non-CTX group (63.9%, 38.4%, and 32.0% in the CTX vs. 48.4%, 17.86%, and 8.9% in the non-CTX groups, respectively; Fig. 2; p=0.015). The disease-free survival and disease-specific survival in the CTX group were better than in the non-CTX group. We conducted Cox regression analysis to identify independent risk factors for survival. The postoperative adjuvant chemotherapy was identified to be independent prognostic factors for disease-specific survival of stage IV (T4N1-3M0 and T1-3N3M0) gastric cancer patients who underwent gastrectomy with curative intent (Table 4).
Fig. 1.
Disease specific survival curve of stage IV gastric cancer with curative gastrectomy according to receiving adjuvant chemotherapy.
Fig. 2.
Disease free survival curve of stage IV gastric cancer patients with curative gastrectomy according to receiving adjuvant chemotherapy.
Table 4.
Multivariate analysis of the prognostic factors for disease-specific survival of stage IV (T4N1-3M0 and T1-3N3M0) gastric cancer patients
Discussion
The purpose of adjuvant chemotherapy is to increase disease-free survival and overall survival in cancer patients by eradicating residual micrometastases. Many clinical trials have been conducted by Japanese and Western investigators over several years (11-15). Three large-scale randomized controlled studies have been conducted to assess the effect of adjuvant chemotherapy on improving survival in patients with potentially curable gastric cancer (12,16,17). However, the studies utilized different methodologies and heterogenous groups with various chemotherapeutic regimens, so postoperative adjuvant chemotherapy was not defined as standard treatment of gastric cancer after curative surgery. Until the present, there is no confirmation which stage of gastric cancer patients has a greater effect from adjuvant chemotherapy than others.
The aim of this study was to support data regarding the benefit of adjuvant chemotherapy in pathologically-determined stage IV gastric cancer patients who underwent curative gastrectomy.
The limitation of this study was that it was conducted based on retrospective data. Although this study was performed retrospectively, we think that the bias may be reduced by the fact that this data results from one surgeon and a single institution.
We used various chemotherapy regimens because new chemotherapy regimens were developed during the period of this study. However, the survival difference in respective chemotherapy regimen showed no statistical significance in this study because of a small number of cases in each regimen. Since the chemotherapy regimens varied in clinical trials, it cannot be concluded which regimen should be used in adjuvant chemotherapy in gastric cancer (6).
In contrast to gastric cancer, adjuvant chemotherapy according to specific stage and high risk factors is recommended for colon cancer (18,19). It is well-established that 5-FU-based adjuvant chemotherapy can increase survival for patients with completely resected stage III colon cancer (21). Gastric cancer has no stage-specific chemotherapy guidelines. A Japanese clinical trial has demonstrated a significant survival benefit for postoperative adjuvant chemotherapy in stages II and III gastric cancer (20). However, we did not identify any clinical trials attempting to reveal an effect of adjuvant chemotherapy on stage IV gastric cancer patients who underwent curative gastrectomy.
The characteristics of patients with adjuvant chemotherapy in this study who showed a clinically better disease-free survival and specific survival were younger patients and gastric cancers with undifferentiated histology compared to patients without adjuvant chemotherapy.
Gastric cancer remains a worldwide health problem accounting for 10% of all new cancer diagnoses and 12% of all cancer-related deaths (22). Although mortality is high, no standard chemotherapy increasing survival is present. In the future, many institutions should conduct randomized controlled trials trying to reveal the effect of stage-specific adjuvant chemotherapy on gastric cancer patients.
Conclusion
Stage-specific adjuvant chemotherapy for gastric cancer may be needed to increase survival. First of all, it should be preceded who can obtain survival benefit from adjuvant chemotherapy prior to attempting chemotherapy. We suggest that adjuvant chemotherapy may be associated with a survival benefit in younger patients with stage IV undifferentiated gastric cancer (T4N1-3M0 and T1-3N3M0) after curative gastrectomy. Many clinical trials should be conducted to figure out the effect of stage-specific adjuvant chemotherapy on gastric cancer.
References
- 1.Parkin DM, Pisani P, Ferlay J. Global cancer statistics. CA Cancer J Clin. 1999;49:33–64. doi: 10.3322/canjclin.49.1.33. [DOI] [PubMed] [Google Scholar]
- 2.Hermans J, Bonenkamp JJ, Boon MC, Bunt AM, Ohyama S, Sasako M, et al. Adjuvant therapy after curative resection for gastric cancer: meta-analysis of randomized trials. J Clin Oncol. 1993;11:1441–1447. doi: 10.1200/JCO.1993.11.8.1441. [DOI] [PubMed] [Google Scholar]
- 3.Panzini I, Gianni L, Fattori PP, Tassinari D, Imola M, Fabbri P, et al. Adjuvant chemotherapy in gastric cancer: a meta-analysis of randomized trials and a comparison with previous meta-analyses. Tumori. 2002;88:21–27. [PubMed] [Google Scholar]
- 4.Liu TS, Wang Y, Chen SY, Sun YH. An updated meta-analysis of adjuvant chemotherapy after curative resection for gastric cancer. Eur J Surg Oncol. 2008;34:1208–1216. doi: 10.1016/j.ejso.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 5.Mari E, Floriani I, Tinazzi A, Buda A, Belfiglio M, Valentini M, et al. Efficacy of adjuvant chemotherapy after curative resection for gastric cancer: a meta-analysis of published randomised trials. A study of the GISCAD (Gruppo Italiano per lo Studio dei Carcinomi dell'Apparato Digerente) Ann Oncol. 2000;11:837–843. doi: 10.1023/a:1008377101672. [DOI] [PubMed] [Google Scholar]
- 6.Zhao SL, Fang JY. The role of postoperative adjuvant chemotherapy following curative resection for gastric cancer: a meta-analysis. Cancer Invest. 2008;26:317–325. doi: 10.1080/07357900701834686. [DOI] [PubMed] [Google Scholar]
- 7.Greene FL. TNM staging for malignancies of the digestive tract: 2003 changes and beyond. Semin Surg Oncol. 2003;21:23–29. doi: 10.1002/ssu.10018. [DOI] [PubMed] [Google Scholar]
- 8.Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655. [PubMed] [Google Scholar]
- 9.Aurello P, D'Angelo F, Rossi S, Bellagamba R, Cicchini C, Nigri G, et al. Classification of lymph node metastases from gastric cancer: comparison between N-site and N-number systems. Our experience and review of the literature. Am Surg. 2007;73:359–366. [PubMed] [Google Scholar]
- 10.Noguchi M, Miyazaki I. Prognostic significance and surgical management of lymph node metastasis in gastric cancer. Br J Surg. 1996;83:156–161. [PubMed] [Google Scholar]
- 11.Di Costanzo F, Gasperoni S, Manzione L, Bisagni G, Labianca R, Bravi S, et al. Adjuvant chemotherapy in completely resected gastric cancer: a randomized phase III trial conducted by GOIRC. J Natl Cancer Inst. 2008;100:388–398. doi: 10.1093/jnci/djn054. [DOI] [PubMed] [Google Scholar]
- 12.Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Fujii M, Nashimoto A, et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007;357:1810–1820. doi: 10.1056/NEJMoa072252. [DOI] [PubMed] [Google Scholar]
- 13.Nashimoto A, Nakajima T, Furukawa H, Kitamura M, Kinoshita T, Yamamura Y, et al. Randomized trial of adjuvant chemotherapy with mitomycin, Fluorouracil, and Cytosine arabinoside followed by oral Fluorouracil in serosa-negative gastric cancer: Japan Clinical Oncology Group 9206-1. J Clin Oncol. 2003;21:2282–2287. doi: 10.1200/JCO.2003.06.103. [DOI] [PubMed] [Google Scholar]
- 14.Bajetta E, Buzzoni R, Mariani L, Beretta E, Bozzetti F, Bordogna G, et al. Adjuvant chemotherapy in gastric cancer: 5-year results of a randomised study by the Italian Trials in Medical Oncology (ITMO) Group. Ann Oncol. 2002;13:299–307. doi: 10.1093/annonc/mdf040. [DOI] [PubMed] [Google Scholar]
- 15.Neri B, Cini G, Andreoli F, Boffi B, Francesconi D, Mazzanti R, et al. Randomized trial of adjuvant chemotherapy versus control after curative resection for gastric cancer: 5-year follow-up. Br J Cancer. 2001;84:878–880. doi: 10.1054/bjoc.2000.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11–20. doi: 10.1056/NEJMoa055531. [DOI] [PubMed] [Google Scholar]
- 17.Macdonald JS, Smalley SR, Benedetti J, Hundahl SA, Estes NC, Stemmermann GN, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345:725–730. doi: 10.1056/NEJMoa010187. [DOI] [PubMed] [Google Scholar]
- 18.Sobrero A. Should adjuvant chemotherapy become standard treatment for patients with stage II colon cancer? For the proposal. Lancet Oncol. 2006;7:515–516. doi: 10.1016/S1470-2045(06)70727-6. [DOI] [PubMed] [Google Scholar]
- 19.Quah HM, Chou JF, Gonen M, Shia J, Schrag D, Landmann RG, et al. Identification of patients with high-risk stage II colon cancer for adjuvant therapy. Dis Colon Rectum. 2008;51:503–507. doi: 10.1007/s10350-008-9246-z. [DOI] [PubMed] [Google Scholar]
- 20.Nakajima T, Kinoshita T, Nashimoto A, Sairenji M, Yamaguchi T, Sakamoto J, et al. Randomized controlled trial of adjuvant uracil-tegafur versus surgery alone for serosa-negative, locally advanced gastric cancer. Br J Surg. 2007;94:1468–1476. doi: 10.1002/bjs.5996. [DOI] [PubMed] [Google Scholar]
- 21.Benson AB, 3rd, Schrag D, Somerfield MR, Cohen AM, Figueredo AT, Flynn PJ, et al. American society of clinical oncology recommendations on adjuvant chemotherapy for stage II colon cancer. J Clin Oncol. 2004;22:3408–3419. doi: 10.1200/JCO.2004.05.063. [DOI] [PubMed] [Google Scholar]
- 22.Verdecchia A, Corazziari I, Gatta G, Lisi D, Faivre J, Forman D. Explaining gastric cancer survival differences among European countries. Int J Cancer. 2004;109:737–741. doi: 10.1002/ijc.20047. [DOI] [PubMed] [Google Scholar]