Abstract
Purpose
The aim of this study was to evaluate the clinicopathological characteristics of uterine leiomyosarcoma (LMS) and possible prognostic factors.
Materials and Methods
This study included 31 patients with histologically proven LMS at Samsung Medical Center and Ajou University Hospital between 1994 and 2007. The medical records and available histological slides were reviewed retrospectively.
Results
The median age was 46 years (range, 32~63). The most common symptom was vaginal bleeding (11 patients, 35.5%). There were 23 patients with stage I, one patient with stage III, seven patients with stage IV disease. The median follow up time was 29 months (range, 1~94). The most common recurrence site was lung (5 case), followed by pelvis and upper abdomen (2 case). Nine patients died of disease with a 5-year overall survival rate of 63%. Early tumor stage and mitotic count were the prognostic factor in univariate analysis (p<0.0001 and p=0.0031, respectively), but early tumor stage only was associated with prognosis in multivariate analysis (p=0.010 vs p=0.143). Adjuvant treatment for early stage disease did not decrease the recurrence rate (p=0.1075), but high mitotic count (15>10HPF) had a trend for disease recurrence in early stage LMS (p=0.0859).
Conclusion
Mitotic count less than 15/HPF in early stage may be related with longer progression-free interval, but we could not reach the conclusion that adjuvant therapy in early stage LMS be effective.
Keywords: Stage, Mitotic count, Adjuvant therapy, Prognostic factor
Introduction
Uterine leiomyosarcoma (LMS) is a rare gynecologic malignancy, comprising about 1% of all uterine malignancies and 25~36% of uterine sarcomas (1-3). LMS is generally considered a highly malignant neoplasm with 5-year survival rate of 18.8% to 65% for all stages of disease (4,5). The most frequent symptoms of uterine LMS are abnormal vaginal bleeding and palpable mass followed by weight loss and weakness (3). Intraoperative visual distinction between leiomyosarcoma and large leiomyoma is nearly impossible (6), therefore leimyosarcoma is often diagnosed at postoperative histologic evaluation of hysterectomy or myomectomy specimens.
While LMS is usually diagnosed at early stage, its prognosis is poor due to frequent recurrence, especially at distant sites. The treatment choice for uterine LMS is surgical removal such as total hysterectomy and bilateral salpingo-oophporectomy, however, surgical staging appears to be less important due to rather frequent hematogenous spread (7).
Adjuvant therapy including chemotherapy and/or radiation therapy has been used to reduce recurrence, but its clinical efficacy is uncertain, especially in early stage (3,8,9).
Different prognostic factors such as age, menopause status, mitotic count, adjuvant therapy have been reported in uterine LMS, but these factors as prognostic factors remain controversial except for tumor stage (3,9).
The aim of this study was to evaluate the clinicopathological characteristics of uterine LMS and possible prognostic factors.
Materials and Methods
This study included 31 patients with histologically proven LMS at Samsung Medical Center and Ajou University Hospital between 1994 and 2007. The medical records were reviewed for demographic information, presenting symptoms, treatment, mitotic count, and survival analysis. Available histological slides were also reviewed by one pathologist (Kim JH). The Institutional Review Board approved this study. LMS was defined histologically by a presence of two of the three following criteria: (1) significant nuclear atypia, (2) greater than 10 mitotic counts per 10 high-power field (HPF), and (3) coagulative tumor cell necrosis (10). The modified 1988 FIGO staging for endometrial cancer was used for this disease.
Total hysterectomy was performed in all patients. However, salpingo-oophorectomy(either bilateral or unilateral) and lymph node dissection were not performed in all patients. The decision to perform adjuvant therapy (chemotherapy or radiation therapy) was based on the attending physician's judgment. Treatment of recurrent disease consisted of chemotherapy and/or cytoreductive surgery if accessible.
All patients were followed up at 3 month's interval during the first 2 years, at 6 month's interval for the following 2 years and annually afterwards. Abdominal and pelvic computed tomograpys (CT) or magnetic resonance imaging (MRI) was performed 6 month's interval during the first two years. In cases of clinical suspicion of recurrence, CT or MRI was repeated regardless of the interval from the previous radiographic imaging.
Correlation between mitosis and stage was tested using Chi-square test. The end point of overall survival (OS) and disease free survival (DFS) were used for analysis. Survival probabilities were calculated by the Kaplan and Meier Method. Univariate analysis was performed using the log-rank test. Cox proportional hazards regression was used to identify independent prognostic factors. p values of less than 0.05 were considered statistically significant.
Results
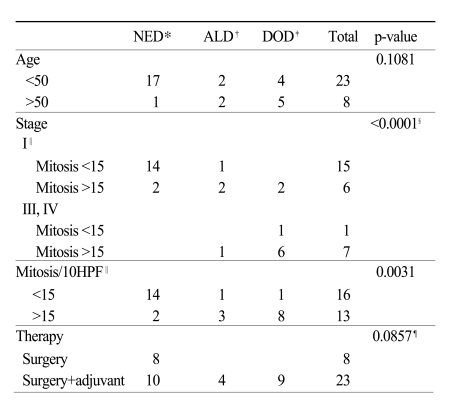
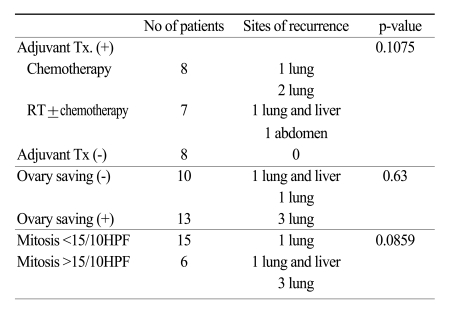
A total of 31 patients with uterine LMS were included in this study. Patient characteristics are summarized in Table 1. The median age was 46 years (range, 32~63). The most common symptom was vaginal bleeding (11 patients, 35.5%). There were 23 patients with stage I, one patient with stage III, seven patients with stage IV disease. Except for stage IV disease, 20 patients had a preoperative impression of uterine leiomyoma and 13 patients out of these had intraoperative diagnosis of leiomyosarcoma by frozen section biopsy. In seven patients, diagnosis was made postoperatively. All patients had total abdominal hysterectomy (TAH) with or without bilateral salpingo-oophorectomy (BSO), and eight patients with stage I disease had pelvic lymphadenectomy, in whom lymph node metastasis was proven pathologically in none. Thirteen patients had preservation of at least one ovary (Table 2). The median follow up time was 29 months (range, 1~94). Five patients with stage I and two patients with stage III and IV had disease recurrences. The most common recurrence site was the lung (five cases), followed by pelvis and upper abdomen (two cases). Six patients with stage IV had persistent disease after initial treatment. Nine patients died of disease with a 5-year overall survival rate of 63% (Fig. 1). The median mitotic count of all patients was 15. Table 2 shows the significant relationship of stage and mitotic count (p=0.01). Patients with stage I disease had 5-year OS rate of 88%, and patients with stage III-IV had 2-year OS of 18% (Fig. 2). Tumor stage and mitotic count were the prognostic factor in univariate analysis (p<0.0001 and p=0.0031, respectively), but tumor stage only was the significant prognostic factor in multivariate analysis. (p=0.010 vs p=0.143) Age, adjuvant therapy and ovarian preservation had no prognostic significance in multivariate analysis.
Table 1.
Patients' characteristics and p-value according to prognostic factors
*no evidence of disease, †alive with disease, ‡dead of disease, §stage I vs stage III, IV, ∥two patients not available for slide review, ¶surgery vs surgery + adjuvant.
Table 2.
Recurrences in 23 patients with stage I leimyosarcoma
Fig. 1.
Overall survival of 31 patients.
Fig. 2.
Survival comparison between patients with early stage and patients with advanced stage (p<0.0001).
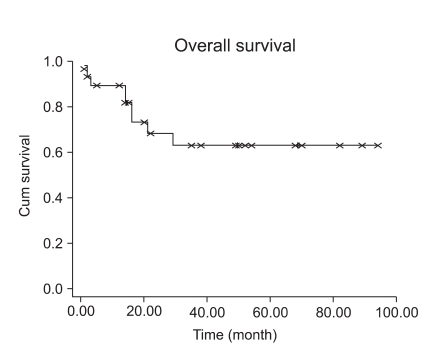
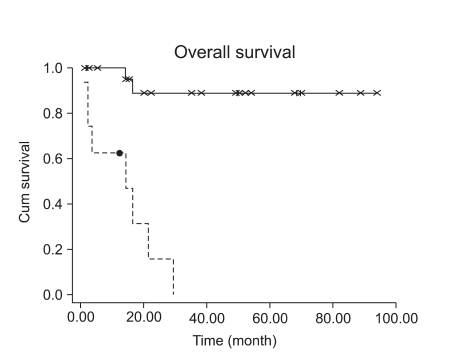
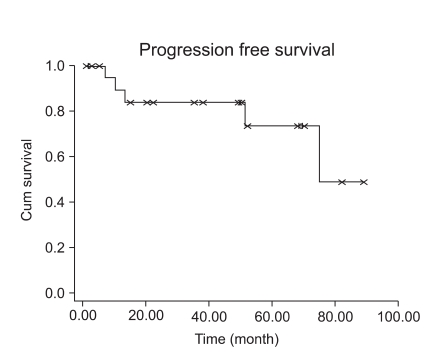
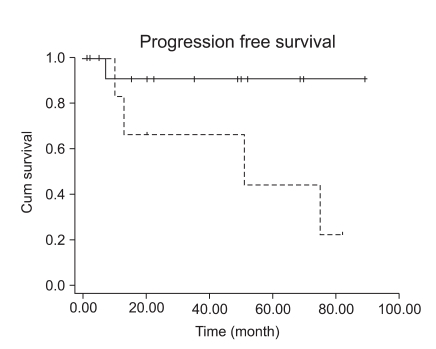
Fifteen patients with stage I LMS (15/23, 65.2%) had adjuvant chemotherapy or radiation therapy. However, adjuvant treatment for early stage disease did not decrease the recurrence rate (p=0.1075). Patients with early stage LMS had 5-year DFS of 73% (Fig. 3). Although high mitotic count (>15/10HPF) was not associated with a progression-free interval in early stage LMS (Fig. 4, p=0.0859), it showed at least a trend for disease recurrence in early stage LMS.
Fig. 3.
Disease free survival in early stage disease (n=23).
Fig. 4.
Survival comparison between patients with high mitotic count and patients with low mitotic count (p=0.0859).
Discussion
Uterine LMS is rare female neoplasms and is considered highly malignant due to high metastatic capacity and recurrence rates even if diagnosed at early stages (2). Although these tumors are usually diagnosed at an early stage, they have the propensity for early vascular space invasion and distant hematogenous metastatis (11,12). The most common distant metastasis occurs in the lung and the abdomen. Our results also showed five cases of lung metastasis out of seven recurrences
At present, TAH and BSO is the only treatment with proven curative value for early stage LMS (13). Our initial treatment for patients with LMS included surgery for all patients. Pelvic lymph node dissection was performed in only eight patients (25.8%), because lymphadenectomy has not been shown to be therapeutically or prognostically significant (14). Our results also showed no lymph node metastasis in all eight cases. Spread of leiomyosarcoma is mainly hematogenous, so surgical staging appears to be less important.
The issue on ovarian preservation in young women with early stage disease is somewhat controversial. According to Giuntoli et al., ovarian preservation did not adversely affect survival (15). We also found that ovarian preservation did not show increased disease recurrence (p=0.63) in early stage disease. Therefore grossly normal ovary may be saved in young patients with early stage LMS.
Several chemotherapeutic agents such as ifosfamide and adriamycin have been found to be effective in sarcomas, but they can be only palliative of disease-related symptoms in advanced or recurrent LMS (16). We also used cisplatin plus adriamycin regimen or cisplatin plus adriamycin plus ifosfamide in advanced stage disease. Although chemotherapy and radiation therapy may induce favorable responses in recurrent cases, these modalities did not exhibit an improvement in disease-specific survival (17).
The influence of mitotic count on prognosis remains controversial. Nola et al. and Blom et al. reported no significant correlation between mitotic count and prognosis (18,19). Larson et al., however, found mitotic count to act as a strong predictor of survival (20). In the Gynecologic Oncology Group (GOG) study, mitotic count was the only factor significantly related with progression-free interval in early stage LMS. In univariate analysis, we found a statistically significant association between mitotic count and overall survival (Fig. 2, p=0.0031), but not in multivariate analysis (p=0.143).
Contrary to the study by Mayerhofer who used more than 10/10HPF as a high mitotic count criteria, high mitotic count in our study (>15/10HPF) was not associated with progression-free interval in early stage LMS (Fig. 4, p=0.0859), but this discrepancy might be probably due to the small number of patients in our study (n=23), and at least we could assume that high mitotic count (>15/10HPF) has a trend for disease recurrence in early stage LMS. Wu also reported a trend toward improved survival in patients with mitotic count less than 15/10 HPF (21). Furthermore, we found a statistically significant interaction between mitotic count and tumor stage (p=0.01).
In our study, 5-year OS rate was 63%, and this is consistent with those of Salazar et al. and Bazzocchi et al., who reported 5-year OS rates of 63% and 62%, respectively (22,23). In both studies, 80% of patients were with stage I tumors, similar to our investigation with a total of 74.2% being with stage I tumors.
Patients with advanced stages (III, IV) had much worse prognosis than patients with early stage, and this result is consistent with reports by others (7,15,21).
The 5-year OS in early stage LMS seems somewhat higher in our study than in the larger study (15), but this is probably because the patients' distribution was skewed toward mitotic count less than 15/10 HPF, and there was only small number of patients in this study. Our study is limited by the relative rarity of this disease, but we could see that early tumor stage still has an important prognostic significance.
The value of adjuvant radiation is controversial. The purpose of post-operative radiation therapy in this study was for improving tumor control in the pelvis, based on the report by Knock et al. (24), who reported 72.4% local control rate in LMS. In this study, fifteen patients (65%) out of 23 stage I LMS received radiation therapy and/or chemotherapy as adjuvant therapy. Nonetheless, five patients (21.8%) had distant site recurrence despite adjuvant therapy. Although there is small number of patients enrolled in our study, there seems to be little evidence that adjuvant therapy changes the disease free survival (8). There was no disease recurrence in patients who received no adjuvant therapy. This would probably be related with the high mitotic count in patients who had disease recurrence. Gadducci et al (25). also reported tumor recurrences of 38% in stage I uterine LMS regardless of adjuvant therapy. Contrary to malignant mixed Mullerian tumor or endometrial stromal sarcoma, adjuvant postoperative radiation therapy was less effective in decreasing local recurrence in LMS because pulmonary metastases are more common than local recurrences (4).
Conclusion
The early tumor stage (i.e., stage I) had a favorable prognosis in uterine LMS. Mitotic count less than 15/HPF may be related with longer progression-free interval in stage I disease, but we could not reach the conclusion that adjuvant therapy in early stage LMS be effective. Continued investigation for other prognostic factors and more effective adjuvant regimen or agent would be necessary for the management of this rare neoplasm.
References
- 1.Echt G, Jepson J, Steel J, Langholz B, Luxton G, Hernandez W, et al. Treatment of uterine sarcomas. Cancer. 1990;66:35–39. doi: 10.1002/1097-0142(19900701)66:1<35::aid-cncr2820660108>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 2.Norris HJ, Zaloudek CJ. In: Mesenchymal tumors of the uterus. 2nd ed. Blaustein A, editor. New York: Springer-Verlag; 1982. [Google Scholar]
- 3.Zaloudek CJ, Norris HJ. In: Mesechymal tumors of the uterus. 4th ed. Kuman RJ, editor. New York: Springer-Verlag; 1993. [Google Scholar]
- 4.Livi L, Paiar F, Shah N, Blake P, Villanucci A, Amunni G, et al. Uterine sarcoma: twenty-seven years of experience. Int J Radiat Oncol Biol Phys. 2003;57:1366–1373. doi: 10.1016/s0360-3016(03)00750-8. [DOI] [PubMed] [Google Scholar]
- 5.Major FJ, Blessing JA, Silverberg SG, Morrow CP, Creasman WT, Currie JL, et al. A Gynecologic Oncology Group study. Prognostic factors in early-stage uterine sarcoma. Cancer. 1993;71(4 Suppl):1702–1709. doi: 10.1002/cncr.2820710440. [DOI] [PubMed] [Google Scholar]
- 6.Leibsohn S, d'Ablaing G, Mishell DR, Jr, Schlaerth JB. Leiomyosarcoma in a series of hysterectomies performed for presumed uterine leiomyomas. Am J Obstet Gynecol. 1990;162:968–974. doi: 10.1016/0002-9378(90)91298-q. [DOI] [PubMed] [Google Scholar]
- 7.Mayerhofer K, Obermair A, Windbichler G, Petru E, Kaider A, Hefler L, et al. Leiomyosarcoma of the uterus: a clinicopathologic multicenter study of 71 cases. Gynecol Oncol. 1999;74:196–201. doi: 10.1006/gyno.1999.5436. [DOI] [PubMed] [Google Scholar]
- 8.Berchuck A, Rubin SC, Hoskins WJ, Saigo PE, Pierce VK, Lewis JL., Jr Treatment of uterine leiomyosarcoma. Obstet Gynecol. 1988;71(6 Pt 1):845–850. [PubMed] [Google Scholar]
- 9.Schwartz Z, Dgani R, Lancet M, Kessler I. Uterine sarcoma in Israel: a study of 104 cases. Gynecol Oncol. 1985;20:354–363. doi: 10.1016/0090-8258(85)90217-3. [DOI] [PubMed] [Google Scholar]
- 10.Kempson RL, Bari W. Uterine sarcomas. Classification, diagnosis, and prognosis. Hum Pathol. 1970;1:331–349. [PubMed] [Google Scholar]
- 11.Fleming WP, Peters WA, 3rd, Kumar NB, Morley GW. Autopsy findings in patients with uterine sarcoma. Gynecol Oncol. 1984;19:168–172. doi: 10.1016/0090-8258(84)90176-8. [DOI] [PubMed] [Google Scholar]
- 12.van Nagell JR, Jr, Hanson MB, Donaldson ES, Gallion HH. Adjuvant vincristine, dactinomycin, and cyclophosphamide therapy in stage I uterine sarcomas. A pilot study. Cancer. 1986;57:1451–1454. doi: 10.1002/1097-0142(19860415)57:8<1451::aid-cncr2820570802>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 13.Giuntoli RL, 2nd, Bristow RE. Uterine leiomyosarcoma: present management. Curr Opin Oncol. 2004;16:324–327. doi: 10.1097/01.cco.0000127721.55676.f6. [DOI] [PubMed] [Google Scholar]
- 14.Goff BA, Rice LW, Fleischhacker D, Muntz HG, Falkenberry SS, Nikrui N, et al. Uterine leiomyosarcoma and endometrial stromal sarcoma: lymph node metastases and sites of recurrence. Gynecol Oncol. 1993;50:105–109. doi: 10.1006/gyno.1993.1172. [DOI] [PubMed] [Google Scholar]
- 15.Giuntoli RL, 2nd, Metzinger DS, DiMarco CS, Cha SS, Sloan JA, Keeney GL, et al. Retrospective review of 208 patients with leiomyosarcoma of the uterus: prognostic indicators, surgical management, and adjuvant therapy. Gynecol Oncol. 2003;89:460–469. doi: 10.1016/s0090-8258(03)00137-9. [DOI] [PubMed] [Google Scholar]
- 16.Sutton G, Blessing JA, Malfetano JH. Ifosfamide and doxorubicin in the treatment of advanced leiomyosarcomas of the uterus: a Gynecologic Oncology Group study. Gynecol Oncol. 1996;62:226–229. doi: 10.1006/gyno.1996.0220. [DOI] [PubMed] [Google Scholar]
- 17.Giuntoli RL, 2nd, Garrett-Mayer E, Bristow RE, Gostout BS. Secondary cytoreduction in the management of recurrent uterine leiomyosarcoma. Gynecol Oncol. 2007;106:82–88. doi: 10.1016/j.ygyno.2007.02.031. [DOI] [PubMed] [Google Scholar]
- 18.Blom R, Guerrieri C, Stal O, Malmstrom H, Simonsen E. Leiomyosarcoma of the uterus: a clinicopathologic, DNA flow cytometric, p53, and mdm-2 analysis of 49 cases. Gynecol Oncol. 1998;68:54–61. doi: 10.1006/gyno.1997.4889. [DOI] [PubMed] [Google Scholar]
- 19.Nola M, Babic D, Ilic J, Marusic M, Uzarevic B, Petrovecki M, et al. Prognostic parameters for survival of patients with malignant mesenchymal tumors of the uterus. Cancer. 1996;78:2543–2550. doi: 10.1002/(sici)1097-0142(19961215)78:12<2543::aid-cncr14>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 20.Larson B, Silfversward C, Nilsson B, Pettersson F. Prognostic factors in uterine leiomyosarcoma. A clinical and histopathological study of 143 cases. The Radiumhemmet series 1936-1981. Acta Oncol. 1990;29:185–191. doi: 10.3109/02841869009126543. [DOI] [PubMed] [Google Scholar]
- 21.Wu TI, Chang TC, Hsueh S, Hsu KH, Chou HH, Huang HJ, et al. Prognostic factors and impact of adjuvant chemotherapy for uterine leiomyosarcoma. Gynecol Oncol. 2006;100:166–172. doi: 10.1016/j.ygyno.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 22.Bazzocchi F, Brandi G, Pileri S, Mancuso A, Massaro A, Martinelli G. Clinical and pathologic prognostic features of leiomyosarcoma of the uterus. Tumori. 1983;69:75–77. doi: 10.1177/030089168306900113. [DOI] [PubMed] [Google Scholar]
- 23.Salazar OM, Bonfiglio TA, Patten SF, Keller BE, Feldstein M, Dunne ME, et al. Uterine sarcomas: natural history, treatment and prognosis. Cancer. 1978;42:1152–1160. doi: 10.1002/1097-0142(197809)42:3<1152::aid-cncr2820420319>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 24.Knocke TH, Kucera H, Dorfler D, Pokrajac B, Potter R. Results of postoperative radiotherapy in the treatment of sarcoma of the corpus uteri. Cancer. 1998;83:1972–1979. doi: 10.1002/(sici)1097-0142(19981101)83:9<1972::aid-cncr13>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 25.Gadducci A, Landoni F, Sartori E, Zola P, Maggino T, Lissoni A, et al. Uterine leiomyosarcoma: analysis of treatment failures and survival. Gynecol Oncol. 1996;62:25–32. doi: 10.1006/gyno.1996.0185. [DOI] [PubMed] [Google Scholar]