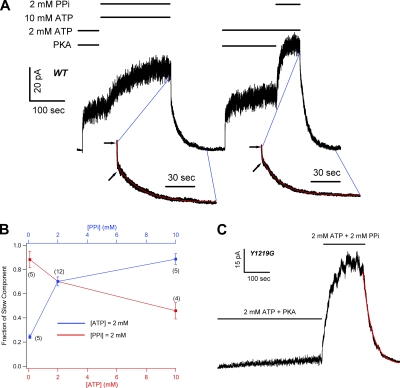
Figure 5.
ATP and MgPPi compete for a common binding site. (A) Steady-state macroscopic current of WT-CFTR activated by 2 mM ATP and PKA was further increased by the application of 2 mM MgPPi plus 2 mM ATP (right) or 2 mM MgPPi plus 10 mM ATP (left). With lower [ATP] in the PPi solution, the current increase proceeds more rapidly (right). Fitting the current relaxation yields two time constants after the removal of 2 mM ATP plus 2 mM PPi (τ1 = 0.55 ± 0.06 s and τ2 = 26.9 ± 1.56 s; n = 12) or after 2 mM MgPPi plus 10 mM ATP (τ1 = 0.34 ± 0.03 s and τ2 = 30.4 ± 3.1 s; n = 4). Arrows indicate the end of the fast component during current decay. (B) The fractional amplitude of the slow component under different combinations of [MgPPi] and [ATP]. Raising [ATP] (red line, lower x axis) or reducing [PPi] (blue line, upper x axis) decreases the fractional amplitude of the slow component. (C) Effects of PPi on Y1219G-CFTR. 2 mM ATP plus PKA activated a small amount of current due to a reduced apparent affinity for ATP by the mutation. Upon application of 2 mM PPi plus 2 mM ATP, the current was greatly enhanced. The current decays monotonically with τ = 30.7 ± 4.5 s (n = 5).