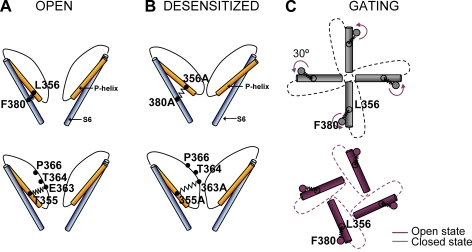
Figure 6.
Illustrated representations of hypothesized molecular interactions between S6 and P helix during gating. (A) In the open (and closed) states of the WT CNGA1 channel, the hydrophobic interaction between Phe380 and Leu356 couples S6 and the P helix, and a presumed H-bond between Thr355 and Glu363 couples the P helix and the pore wall. (B) In mutant channels E363A, T355A, L356A, and F380A, these interactions are lost and, after opening, mutant channels desensitize. In mutant channel E363A, residues in positions 364 and 366 become more accessible to the reagents added to the extracellular medium. (C) A simplified model of gating in CNGA1 channels: in the closed state (gray) the tip of P helices occludes the pore lumen; during gating, the S6 helix rotates by 30° anticlockwise, the pore is not occluded (red), and ions can permeate through it. The hydrophobic interaction between Phe380 and Leu356 couples the rotation of S6 to the motion of the P helix.