Abstract
The C-type lectin-like receptor CD161, which has recently been described to promote T cell expansion, is expressed on a discrete subset of human CD4 T cells. The function of such cells, however, has remained elusive. We now demonstrate that CD161+ CD4 T cells comprise a circulating and gut-resident T helper 17 (Th17) cell population. During Crohn's disease (CD), these CD161+ cells display an activated Th17 phenotype, as indicated by increased expression of interleukin (IL)-17, IL-22, and IL-23 receptor. CD161+ CD4 T cells from CD patients readily produce IL-17 and interferon γ upon stimulation with IL-23, whereas, in healthy subjects, priming by additional inflammatory stimuli such as IL-1β was required to enable IL-23–induced cytokine release. Circulating CD161+ Th17 cells are imprinted for gut homing, as indicated by high levels of CC chemokine receptor 6 and integrin β7 expression. Supporting their colitogenic phenotype, CD161+ Th17 cells were found in increased numbers in the inflammatory infiltrate of CD lesions and induced expression of inflammatory mediators by intestinal cells. Our data identify CD161+ CD4 T cells as a resting Th17 pool that can be activated by IL-23 and mediate destructive tissue inflammation.
CD4 T cells that produce IL-17 (IL-17A) have been recognized as a Th cell lineage distinct from Th1 and Th2, termed Th17 (1). Th17 cells are critical mediators of destructive tissue inflammation in several animal models of chronic inflammation (2). Cytokines typically produced by Th17 cells, such as IL-17, IL-17F, and IL-22, are also found in elevated levels in several human inflammatory diseases (3). Genetic evidence for a putative etiological link of Th17 with human disease emerged from the association of polymorphisms in the IL-23R to altered susceptibility to inflammatory bowel disease (IBD), psoriasis, and ankylosing spondylitis (4). The IL-23R can be expressed on a variety of cells (5) but is most characteristically associated with cells of the Th17 lineage (2, 6). Recently, T cell clones displaying characteristics of Th17 cells could be propagated from disease-affected sites in psoriasis, rheumatoid arthritis, and Crohn's disease (CD) (7, 8). Comparison of the Th17 clones with Th1 and Th2 clones obtained from the same inflammatory infiltrate confirmed Th17-specific expression of factors such as IL-23R, the transcription factor RORγT, and the chemokine receptor CCR6. However, because in vitro–expanded T cell clones were analyzed, it has remained unclear how Th17 cells are involved in the disease process.
In addition to previously recognized Th17 hallmarks, analyses of such Th17 clones revealed specific expression of CD161 (9). CD161 is a C-type lectin-like receptor that is also expressed on subsets of NK and CD8 T cells (10). CD161 has been described as a coactivating receptor promoting antigen-dependent T cell proliferation upon engagement via its recently identified ligand PILAR (11). In adults, CD161+ CD4 T cells are present in the intestine and in the circulation of healthy people (12, 13) but were also found in rheumatic joints and psoriatic lesions (14, 15). Recently, it was shown that CD161+ T cells from the inflammatory infiltrate in psoriasis and CD were enriched for IL-17 producers (9). CD, one of the two most common forms of IBD, displays a particularly strong connection to Th17 inflammation; genome-wide studies not only associated polymorphisms in the IL-23R with increased disease susceptibility but also single nucleotide polymorphisms in additional components of the Th17 pathway (i.e., stat3, p40, jak2, and ccr6) (16). CD is believed to be the result of an aberrant response of the gut-associated lymphoid tissue to bacterial and dietary antigen. Although the presence of Th17 cells in the inflamed intestine has been demonstrated (7–9), their contribution to the disease process has remained elusive.
CD4+ T cells comprise the major lymphocyte subset in the normal intestinal lamina propria and mostly display a memory/effector phenotype (17), with a discrete subset expressing CD161 (12, 18). In light of the proposed link of CD161 with IL-17–producing cells (9), we hypothesized that, similar to observations in mice (19), a gut-resident Th17 population also exists in humans, and set out to (a) identify the physiological Th17 cell pool and (b) characterize the involvement of these inflammatory cells in human disease. By examining surgical gut resections and blood specimens from CD patients and non-IBD controls, we identify the CD161+ population of CD4 T memory cells in the intestine and circulation to express hallmarks of the Th17 pathway (i.e., IL-23R, RORγT, IL-17, and CCR6). Furthermore, we define their contribution to systemic and local inflammation during CD, and demonstrate that these cells can be activated by IL-23 in an inflammatory environment to release proinflammatory cytokines. Thus, our data show that CD161+ CD4 T cells comprise the physiological Th17 cell pool that can be activated by IL-23 during inflammation.
RESULTS AND DISCUSSION
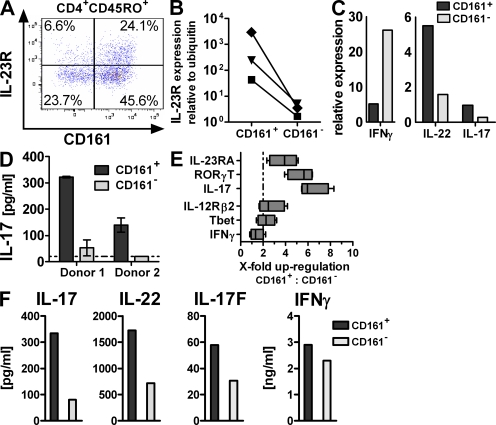
The intestine harbors a large fraction of the body's T cells. To investigate if the human intestinal T cells comprise a population of Th17 cells, as observed in mice, we isolated the lamina propria mononuclear cell (LPMC) fraction from freshly obtained normal adjacent colonic tissue derived from surgery for colorectal cancer or diverticulosis, and analyzed the T helper cell compartment. As expected, the majority of lamina propria CD4 T cells displayed a memory/effector phenotype (CD45RO+, CD45RA−; SD = 73.4 ± 9.2%; Fig. S1 A, available at http://www.jem.org/cgi/content/full/jem.20081712/DC1) and contained a significant proportion of cells expressing CD161 (SD = 64.3 ± 12.1%; Fig. S1 B). CD161+ CD4 T cells exclusively exhibit a memory/effector phenotype (CD45RO+, CD45RA−; Fig. S1 C). Analysis of the CD4 T cell compartment for IL-23R expression, a hallmark of Th17 cells, revealed that CD161+ CD4 T memory cells contained a higher frequency of cells positive for IL-23R than their negative counterparts (Fig. 1 A). Transcriptional analysis of the CD161+ and CD161− CD4 T memory cell subsets after FACS purification (Fig. S1 D) confirmed the flow cytometry results, revealing >10-fold higher levels of il23r expression in CD161+ cells (Fig. 1 B). In addition, CD161+, as compared with CD161−, CD4 T memory cells expressed 3–10-fold higher levels of mRNA for IL-17 and IL-22 but not IFN-γ (Fig. 1 C). Consistent with their transcriptional Th17 signature, CD161+ CD4 T memory cells were the major source of IL-17 protein in the intestine (Fig. 1 D).
Figure 1.
CD161 expression identifies tissue-resident and circulating Th17 cells. (A–D) LPMCs were isolated from non-CD colon specimens. (A) Flow cytometric analysis for IL-23R expression gated on CD45RO+CD4+ T cells. The plot is representative of five independent donors. (B–D) CD4+CD45RO+ T memory cells were FACS sorted for CD161 expression. il23r (B; data are from three individual donors) or il17, il22, and ifng (C; data are representative of two donors) expression was assessed by qRT-PCR and normalized to ubiquitin. Cells were cultured for 3 d with anti-CD2/anti-CD3/anti-CD28 activation beads for measurement of IL-17 production (D; data are from two different donors). The dashed line indicates the lower limit of detection. (E and F) PBMCs from healthy donors were FACS sorted for CD161+ and CD161− CD4+CD25−CD45RA− T memory cells, and gene expression was profiled (E; data are from four different donors; the dashed line indicates twofold change). The boxes indicate interquartile range with median, and whiskers define minimum to maximum values. Cytokine production was determined after 3 d of culture with anti-CD2/anti-CD3/anti-CD28 activation beads. (F; data are representative of at least three donors from independent experiments).
CD161+ CD4 T cells can also be found in the circulation, where they comprise ∼20% of CD4 T lymphocytes, and, similar to the intestine, exhibit a memory phenotype (13). According to a recent study, IL-17-production is attributed to such CD161+ CD4 T cells when compared with total CD161− CD4 T cells (9). To confirm the Th17 nature of circulating CD161+ CD4 T cells, we selectively focused on CD25-depleted memory CD4 T cells to avoid enrichment for naive and regulatory T cells in the CD161− fraction. Indeed, elevated levels of il17, rorc, and il23r transcripts were found in CD161+ as compared with CD161− CD4+CD25−CD45RA− T memory cells (Fig. 1 E). Expression of commonly Th1-associated factors il12rb2, ifng, and tbx21 (the gene encoding T-bet) did not differ significantly between the two subsets (Fig. 1 E). A Th17 phenotype was also reflected in the TCR-mediated cytokine production pattern of CD161+ CD4 T memory cells (Fig. 1 F), indicating that circulating and gut-resident CD161+ CD4 T memory cells constitute a peripheral Th17 pool.
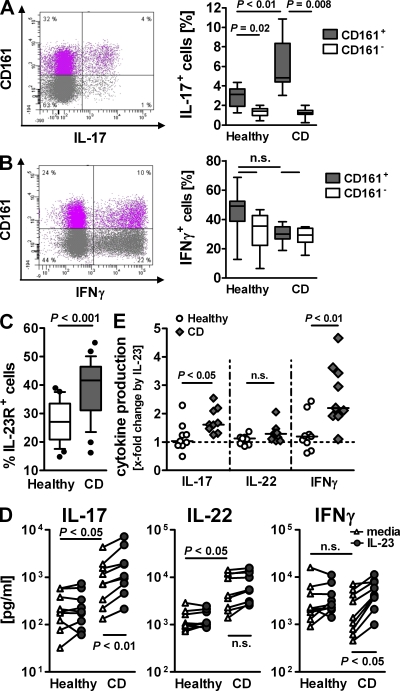
Tracking the phenotype of CD161+ CD4 T cells thus enabled us to follow the involvement of Th17 in CD inflammation. We observed markedly increased IL-17 production in the blood of CD patients, which was confined to CD161+ cells (Fig. 2 A). IFN-γ–producing cells were present in similar levels in both T memory cell subsets (Fig. 2 B). Cells expressing both IL-17 and IFN-γ were only found among CD161+ CD4 T memory cells as a minor fraction, with no significant difference between health and disease (Fig. S2, available at http://www.jem.org/cgi/content/full/jem.20081712/DC1). The enhanced IL-17 production in CD161+ cells was accompanied by a significant increase of IL-23R+ cells (Fig. 2 C). Culturing CD161+ CD4 T memory cells from normal or CD donors confirmed their altered activation state during disease: TCR-mediated IL-17 and IL-22 but not IFN-γ production was increased in CD161+ cells from CD patients (Fig. 2 D). Addition of IL-23 induced a 1.6-fold median increase in IL-17 production and a 2.2-fold median increase in IFN-γ production in CD patients while minimally affecting cytokine levels in healthy donors (1- and 1.2-fold, respectively; Fig. 2 E).
Figure 2.
Activated Th17 phenotype in CD161+ CD4 T memory from CD patients. PBMCs from healthy donors and CD patients were isolated. (A and B) CD4+CD25−CD45RA− T memory cells were FACS purified and cultured with PMA and ionomycin for 4.5 h, and were then stained for CD161 and IL-17 (A) or IFN-γ (B). FACS plots show cytokine production in relation to CD161 expression from one representative CD case. Box plots present cytokine-positive cells as a percentage of CD161+ or CD161− CD4 T memory cells. Boxes show the interquartile range (the whiskers show minimum to maximum; data are from eight different donors). (C) Flow cytometric analysis of CD161+ CD4+CD45RO+ T cells for IL-23R expression. The box plot shows the frequency of IL-23R+ cells determined as the mean value of two separate stains per donor. The boxes show data from 25 different donors as an interquartile range, with whiskers from the 10th to the 90th percentile (outliers are displayed as dots). (D) CD161+ CD4+CD45RA−CD25− T memory cells from healthy and CD donors were cultured for 3 d with anti-CD2/anti-CD3/anti-CD28 beads with or without IL-23. Supernatant was assessed for IL-17, IL-22, and IFN-γ. Data are from nine different donors, and lines connect data points representing the same donor. (E) IL-23–induced x-fold increase in cytokine production by CD161+ CD4+CD45RA−CD25− T memory cells from healthy and CD donors calculated from the data depicted in D. The dashed line indicates baseline at onefold change.
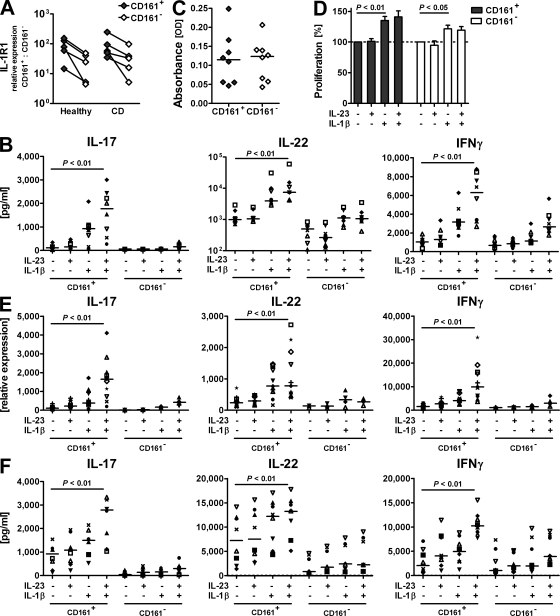
The distinct IL-23 responsiveness in purified CD161+ cells from CD patients suggested that the inflammatory microenvironment provides crucial cosignals for IL-23. Corroborating the notion of IL-1β, particularly, as an important co-stimulator of differentiated Th17 cells (20), CD161+ CD4 T memory cells express higher levels of the IL-1β receptor IL-1R1 than their CD161− counterparts in both healthy and CD donors (Fig. 3 A). Stimulation of normal CD161+ CD4 T memory cells with IL-1β drove IL-17, IL-22, and IFN-γ production, which was, particularly for IL-17 and IFN-γ, further amplified by the addition of IL-23 (2.4- and 1.8-fold mean increase, respectively; Fig. 3 B). In CD161− CD4 T memory cells, co-stimulation with IL-1β and IL-23 failed to induce significant levels of theses cytokines, underlining the unique responsiveness of CD161+ CD4 T memory cells to Th17-promoting signals.
Figure 3.
IL-23 drives IL-17 and IFN-γ production by CD161+ Th17 cells during inflammation. CD161+ and CD161− CD45RA−CD25−CD4+ T memory cells were FACS purified from PBMCs from healthy and CD donors. (A) Expression levels of il1r1 were determined by qRT-PCR and normalized to ubiquitin; data are from five different donors. Connecting lines indicate data points referring to the same donor. (B–F) Cells from healthy (B–E) or CD (F) donors were cultured for 3 d with anti-CD2/anti-CD3/anti-CD28 beads only or with the addition of IL-23 and/or IL-1β as indicated. (B) Protein levels for IL-17, IL-22, and IFN-γ in culture supernatant from normal CD4 T memory cells. Data shown from 8 donors are representative of 15 donors from independent experiments. (C and D) Frequency of viable cells was measured in a colorimetric assay based on enzymatic activity. Cell viability after culture without cytokine stimulation (C) was set as the 100% value to calculate cytokine-induced proliferative responses per donor (D). The bar diagram represents mean values for the eight different donors depicted in C (error bars = SD). The dashed line indicates baseline proliferation in cultures without cytokine stimulation set as 100% value. (E) Transcriptional expression of il17, il22, and ifng in T cells from healthy donors was assessed by qRT-PCR. CD161+ cells from 11 different donors were analyzed with corresponding analysis for CD161− cells from 4 of those donors. (F) IL-17, IL-22, and IFN-γ protein levels in culture supernatant from CD patients' T cells. Data are shown for eight different donors. IL-17 levels for CD subject no. 8 exceeded the scale (values for CD161+ cells with values for CD161− cells in brackets: media, 4,298 [497] pg/ml; IL-23, 7,198 [1,248] pg/ml; IL-1β, 7,943 [946] pg/ml; and IL-23 + IL-1β, 10,911 [1,386] pg/ml). Individual symbols represent individual donors to visualize trends per donor (B, E, and F). Horizontal bars represent medians (B, C, E, and F).
To understand the basis of IL-1β and/or IL-23 actions, we assessed the effect on cell survival and proliferation. The rate of apoptosis was similar across all culture conditions (SD = 7.1 ± 1.7%; not depicted). In addition, there was no difference in the intrinsic proliferative capacity between CD161+ and CD161− CD4 T memory cells (Fig. 3 C). Addition of IL-1β, however, had a significant though modest effect on the proliferation of CD161+ and, to a lesser extent, CD161− CD4 T cells (Fig. 3 D). Interestingly, IL-23 neither added to these effects nor did it induce proliferation by itself (Fig. 3 D). To address whether the increase in cell number was responsible for the increased cytokine production after IL-1β stimulation, we measured the relative expression of il17, ifng, and il22 genes, which is independent of cell number. The overall trend of cytokine transcript expression resembled the pattern of protein levels; if anything, the effect of IL-23, particularly, on IL-17 expression by IL-1β–primed CD161+ CD4 T cells was more pronounced on the transcriptional level (4.4-fold mean increase; Fig. 3 E; and Fig. S3, available at http://www.jem.org/cgi/content/full/jem.20081712/DC1). Thus, although IL-1β affects proliferation and cytokine production, IL-23 adds to the final differentiation of primed Th17 cells by fine tuning their cytokine expression profile. Accordingly, cytokine production in CD161+ CD4 T memory cells from CD patients was most pronounced after stimulation with IL-23 in conjunction with IL-1β (Fig. 3 F).
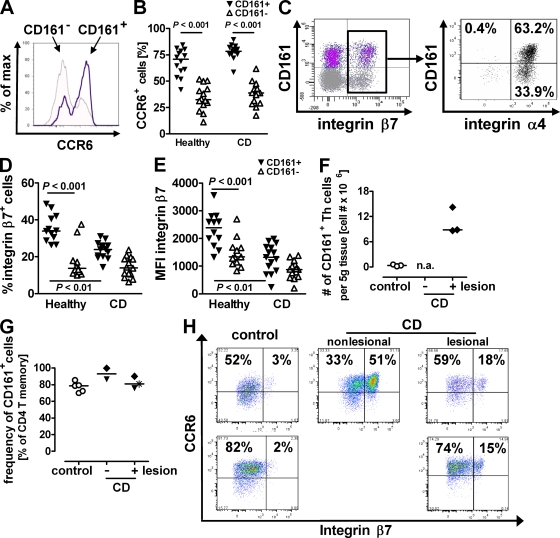
Having shown that CD161+ Th17 cells can be potently activated in an inflammatory environment, we now explored their role in sustaining end-organ inflammation, as defined by their potential to (a) migrate to the disease-affected sites and (b) induce local tissue inflammation. Cell trafficking from the blood into areas of active inflammation requires the expression of the appropriate homing and chemokine receptors. In particular, the chemokine receptor CCR6 facilitates CD4 T memory cell trafficking to epithelial sites (21), and its sole chemokine ligand, CCL20 (MIP-3α), is highly expressed at sites of CD inflammation (22). In healthy subjects, a mean of 67.9% (±12.3% SD) of CD161+ CD4 T memory cells was positive for CCR6 as compared with 34.6% (±12% SD) of their CD161− counterparts (Fig. 4 A). The proportion of CCR6 expression was not altered during disease: CD161+ cells maintained high expression of this receptor, whereas CD161− cells failed to up-regulate it (Fig. 4 B). Because surface expression of CCR6 has recently been attributed to Th17 cells (7, 23), these results not only confirm the inflammatory potential but also the Th17 phenotype of CD161+ CD4 T memory cells.
Figure 4.
CD161+ Th17 cells are imprinted for gut homing and contribute to the inflammatory infiltrate in CD. (A–E) PBMCs from healthy donors and CD patients were isolated and analyzed by flow cytometry. The proportion of CCR6+ cells of normal (A and B) and CD (B) CD161+ and CD161− CD4+CD45RO+ T memory cells. Stains were performed in duplicates, and the mean value per donor from 14 different healthy and CD donors is shown. (C) Distribution of integrin β7+ cells gated on total normal CD4+CD45RA− T memory cells (left), and distribution of integrin α4+ cells gated on total normal integrin β7+ CD4+CD45RA− T memory cells (right; data are representative of eight different donors). (D and E) Frequency (D) or mean fluorescence intensity (MFI; E) of integrin β7+ cells of either CD161+ or CD161− CD4+CD45RA− T memory cells from healthy donors and CD patients; data are from 12 different healthy and 14 different CD donors. (F–H) LPMCs were isolated from CD and control patients' colon resections. The total number per full thickness tissue weight (F) and frequency (G) of CD161+ CD4 T cells were determined by flow cytometry. n.a., no tissue weight available. (H) flow cytometric analysis of LPMCs for CCR6 and β7-integrin expression gated on CD161+ CD4+CD45RA− T memory cells. Plots show data from three different experiments with two different control and two different CD cases, and nonlesional tissue available from one CD patient. Horizontal bars represent medians (B and D–G).
When primed by intestinal DCs, T cells up-regulate the gut homing receptor integrin β7 (24). CD161+ CD4 T cells from normal blood manifested a higher frequency of integrin β7+ cells than CD161− T cells, indicating the intestine as their initial site of priming (Fig. 4 C). Moreover, such integrin β7+ CD161+ CD4 T memory cells coexpressed integrin α4 (Fig. 4 C). Integrin β7, when in complex with integrin α4, binds the gut-specific mucosal addressin cellular adhesion molecule 1 (MAdCAM-1) (25). During CD, MAdCAM-1 is up-regulated in the intestinal vasculature, potentiating the recruitment of integrin α4β7+ cells to disease-affected sites (26). In this context, it was intriguing that in CD patients the frequency of circulating integrin β7+ CD161+ CD4 T cells was notably decreased (Fig. 4 D). The hereby suggested sequestration of such cells in the intestine was supported by another finding: CD161+ CD4 T memory cells from healthy donors presented a higher per cell ratio of integrin β7 than CD161− cells (Fig. 4 E). In CD patients, however, the remaining integrin β7 expressers displayed a significant reduction in the mean fluorescent intensity of integrin β7 staining (Fig. 4 E). It has been previously reported that binding to MAdCAM-1 is favored by dense expression of integrin β7 (27), indicating that integrin β7bright CD161+ CD4 T cells preferentially partition to the intestine during disease.
To investigate whether CD161+ T cells indeed contribute to CD inflammation, we analyzed the composition of the intestinal inflammatory infiltrate. Mononuclear cell isolations from CD gut resections revealed a 20-fold increase of CD161+ CD4 T cells as compared with control specimens (Fig. 4 F). Moreover, these CD161 expressers presented the predominant subset of infiltrating CD4 T cells (Fig. 4 G). Because the gut physiologically contains a high frequency of CD161+ CD4 T cells, we assessed these cells for phenotypical differences in health and disease. The frequency of intestinal integrin β7+ CD161+ CD4 T memory cells was dramatically increased during CD (Fig. 4 H), consistent with the hypothesis that recirculating cells contribute to the increase in total numbers. Interestingly, all such integrin β7+ cells coexpressed CCR6, indicating the Th17 nature of the infiltrate (Fig. 4 H).
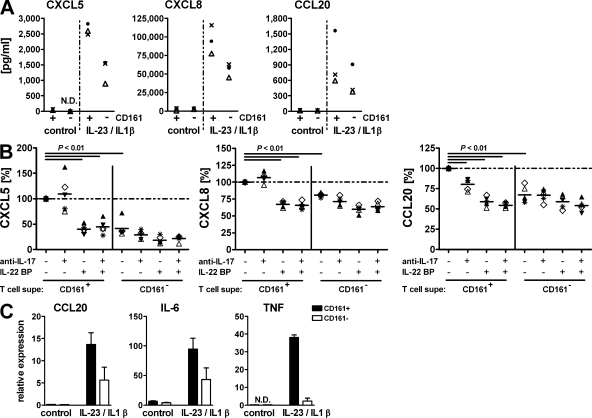
Th17-derived cytokines, including IL-17, IL-17F, and IL-22, have been shown to induce expression of inflammatory mediators by intestinal cells (28). Given the selective association of CD161+ CD4 T cells with the gut, we then asked whether these cells were particularly biased toward inducing intestinal tissue inflammation. Treatment of HT-29 colon epithelial cells with supernatant from IL-1β– and IL-23–activated CD161+ cells compared with CD161− cells led to increased expression of CXCL5, CXCL8, and CCL20, chemokines known to sustain the inflammatory process during CD (Fig. 5 A). CD161+ CD4 T cell–induced chemokine expression was significantly reduced after neutralization of IL-22 and, in the case of CCL20, IL-17 activity, and reached levels similar to CD161− T cell–induced expression (Fig. 5 B). Interestingly, neither recombinant IL-17 or IL-22, nor the combination of both, was sufficient to induce detectable levels of any of those factors (unpublished data). Thus, our results demonstrate that Th17 cytokines define the inflammatory potential of CD161+ CD4 T cells but that these molecules have to act in concert with the inflammatory microenvironment. The even broader implication of CD161+ CD4 T cells in mediating the inflammatory response became evident when we analyzed another cell type, 18Co intestinal myofibroblasts, for their response to activation with T cell supernatant: in addition to CCL20, these cells also expressed elevated levels of IL-6 and TNF-α, two key mediators of tissue destruction in CD (Fig. 5 C).
Figure 5.
Activated CD161+ Th17 cells promote intestinal inflammation. CD161+ and CD161− CD4+CD45RA−CD25− T memory cells were purified from normal PBMCs. Cells were cultured for 3 d in the presence of anti-CD2/anti-CD3/anti-CD28 activation beads with or without IL-23 and IL-1β. Cell-free supernatant was used to stimulate HT-29 colonic epithelial cells (A and B) or 18Co colonic myofibroblasts (C). (A) Chemokines in HT-29 supernatant were measure by ELISA. Data for three T cell donors are shown as representative of eight different donors. (B) Supernatant from IL-23/IL-1β–activated T cells was used to stimulate HT-29 cells. Where indicated, the supernatant was pretreated with monoclonal anti–IL-17 and/or IL-22BP to neutralize the respective cytokine. Chemokine levels after stimulation with untreated supernatant from CD161+ CD4 T memory cells were set as the100% value for each donor (dashed line), and expression for all other stimulations is shown as the percentage thereof. Individual symbols refer to individual donors. Data show five T cell donors from three different experiments. Horizontal bars represent medians. (C) Transcriptional expression of CCL20, IL-6, and TNF in stimulated 18Co cells was measured by qRT-PCR and is depicted as the mean and SD for two different T cell donors.
In conclusion, we have identified a CD161-expressing subpopulation of circulating and gut-resident CD4 T memory cells with a unique ability to respond to IL-23 to become fully differentiated/activated Th17 cells, and demonstrate their central involvement in chronic target-organ inflammation (Fig. S4, available at http://www.jem.org/cgi/content/full/jem.20081712/DC1). Recently, Cosmi et al. isolated CD161+ CD4 T cells from umbilical cord blood as well as postnatal thymus, and demonstrated their early commitment to the Th17 lineage (9). It is conceivable that these cells present precursors of the herein described CD161+ T memory cell population, which during the development of the immune system mature and as precommitted CD161+ Th17 cells selectively partition to the intestine, where they eventually present a distinct Th cell subset. Devoid of an inflammatory environment, these CD161+ T cells are protected from the critical impact of IL-23 activation. Introduction of inflammatory stimuli, however, releases this inhibition: a single, potent proinflammatory cytokine (IL-1β) was able to license purified CD161+ CD4 memory cells for activation by IL-23. Importantly, CD161+ T cells isolated from CD patients' blood displayed an enhanced Th17 phenotype and were capable of responding to IL-23 in the absence of any inflammatory co-stimulus. It will be interesting to decipher whether this indicated an in situ activation before isolation or rather a disease-specific intrinsic defect leading to inappropriate IL-23 responsiveness.
The factors involved in Th cell differentiation and in shaping their effector functions leave a lineage-specific fingerprint on the surface. In these regards, phenotypical characterization of cytokine and chemokine receptor expression has been instrumental in defining Th cell subsets and understanding their biology. In addition, the differential expression of TIM family members on Th1 and Th2 cells (29), as well as the Th2-specific prostaglandin receptor CRTh2, illustrate the complexity of lineage-controlling signals (30). The identification of CD161, a C-type lectin-like receptor with homology to CD69, points at additional co-stimulatory pathways in the Th17 response: the role of CD161 in supporting activation-induced T cell expansion paired with its selective expression on Th17 cells could potentially make it an attractive therapeutic target in CD and other related diseases.
MATERIALS AND METHODS
Patients and blood donors.
Fresh colon specimens were obtained from patients undergoing surgery for CD or noninflammatory diseases such as colorectal cancer or diverticulosis from the University of Michigan (reviewed and approved by the University of Michigan Institutional Review Board), the Cooperative Human Tissue Network at Vanderbilt University (reviewed and approved by Independent Review Consulting), or Asterand (all studies through which Asterand acquires human tissue specimens are approved and overseen by either local or central institutional review boards, and are reviewed by Asterand's Quality Assurance Department). All samples were collected from surplus surgical material with fully informed patient consent. Tissues were reexamined by a pathologist. Blood was obtained from CD patients and healthy individuals scheduled for a routine colonoscopy at the Mayo Clinic (approved by the Mayo Clinic Institutional Review Board), as well as healthy adult volunteer donors participating in the Stanford Medical School Blood Center blood donation program, in accordance with the Declaration of Helsinki. For CD samples, patients with a known history of CD were enrolled, and activity at the time of blood draw was assessed by colonoscopy, followed by pathological examination of biopsies.
Isolation of mononuclear cells.
LPMCs were isolated from surgical colon resections according to an established protocol (31) modified to not exceed 6 h. In brief, isolated mucosa was incubated in HBSS-CMF containing 3 mM βME to dissolve mucus, followed by repeated incubations with 5 mM EDTA in HBSS-CMF to dissociate the epithelial layer. Washed lamina propria was then cut into small pieces and digested during repeated incubations in collagenase and DNase containing complete RPMI 1640 at 37°C in a shaking water bath. Cells were used for FACS analysis and gene expression profiling or cultured overnight, and the nonadherent cell fraction was further processed for T cell subset purification. LPMCs were cultured overnight and the nonadherent cell fraction was further processed. PBMCs were isolated as previously described (6).
Flow cytometry and cell sorting.
Surface expression analysis of LPMCs and PBMCs was performed according to standard protocols using antibodies from BD, except for anti–IL-23R (biotinylated polyclonal goat anti–human; R&D Systems), with streptavidin–Pacific blue (BD) as secondary reagent. For intracellular cytokine staining, CD4+CD45RA−CD25− T memory cells were FACS sorted from healthy and CD PBMCs on a FACSAria (BD), and were stimulated with PMA and ionomycin in the presence of GolgiPlug (BD) for 4.5 h. Cells were permeabilized using Cytofix/Cytoperm reagents (BD) and were incubated with anti–IFN-γ (BD) and anti–IL-17A (eBioscience). CD161+ and CD161− cells were purified from total LPMCs or PBMCs in a two-way sort gated on CD4+CD45RO+ (LPMCs) or CD4+CD25−CD45RA− (PBMCs) T memory cells using a FACSAria.
Cell culture.
T cells were cultured with anti-CD2/anti-CD3/anti-CD28 activation beads for 3 d, as previously described (6). For certain experiments, IL-23 and IL-1β were added at 50 ng/ml. The intestinal cell lines HT-29 and 18Co (American Type Culture Collection) were cultured with 10% T cell supernatant for 1 d. Neutralization of IL-17 and/or IL-22 function was achieved by preincubation of T cell supernatant for 1 h with monoclonal anti–IL-17A and/or IL-22BP (both from R&D Systems).
Cytokine measurement and assessment of viability.
IFN-y, IL-17, IL-22, CXCL5, and CXCL8 were measured using Quantikine kits (R&D Systems). An IL-17F electrochemiluminescence assay was developed in-house for the Meso Scale Discovery (MSD) platform, as previously described (6). Simultaneous detection of IL-17 and IFN-γ in the culture supernatant of CD patients' T cells was performed by electrochemiluminesence in a custom MSD multiplex assay. Apoptosis was measured by Annexin V and propidium iodide staining (Apoptosis Kit; BD). Proliferation was assessed using a CellTiterAQueous kit (Promega).
Gene expression profiling.
RNA was isolated from cell pellets and gene expression was profiled by quantitative RT-PCR (qRT-PCR), as previously described (6). The absence of genomic DNA contamination was confirmed using primers recognizing genomic DNA in the cd4 promoter region. Gene expression data were normalized to the expression of ubiquitin (UBB).
Statistical analysis.
For two-group comparisons, the Mann-Whitney rank-sum test or the Wilcoxon signed-rank test was used. One way analysis of variance was performed using the Kruskal-Wallis statistics test or the Friedman test, both followed by Dunn's post test.
Online supplemental material.
Fig. S1 depicts flow cytometric analysis of LPMCs showing that the majority of colonic CD4 T cells express CD45RO and CD161. Fig. S2 shows that the small proportion of IL-17 and IFN-γ double producers in the circulation is contained within the CD161+ CD4 T memory cell compartment. Fig. S3 shows the x-fold increase of IL-1β–induced cytokine transcript or protein expression by CD161+ Th17 cells after stimulation with IL-23. Fig. S4 summarizes the herein reported findings in an illustration proposing a model for the central role of CD161+ Th17 cells in promoting intestinal inflammation. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20081712/DC1.
Acknowledgments
We thank S. Jungers and F. Shen for technical assistance with flow cytometry–based cell sorting. The many helpful discussions with J. Phillips and D. Cua were much appreciated throughout the study.
Schering-Plough Biopharma (formerly DNAX) is fully funded by the Schering-Plough Corporation. The authors have no further conflicting financial interests.
References
- Kastelein R.A., Hunter C.A., Cua D.J. 2007. Discovery and biology of IL-23 and IL-27: related but functionally distinct regulators of inflammation.Annu. Rev. Immunol. 25:221–242 [DOI] [PubMed] [Google Scholar]
- McGeachy M.J., Cua D.J. 2008. Th17 cell differentiation: the long and winding road.Immunity. 28:445–453 [DOI] [PubMed] [Google Scholar]
- Tesmer L.A., Lundy S.K., Sarkar S., Fox D.A. 2008. Th17 cells in human disease.Immunol. Rev. 223:87–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho J.H. 2008. The genetics and immunopathogenesis of inflammatory bowel disease.Nat. Rev. Immunol. 8:458–466 [DOI] [PubMed] [Google Scholar]
- Parham C., Chirica M., Timans J., Vaisberg E., Travis M., Cheung J., Pflanz S., Zhang R., Singh K.P., Vega F., et al. 2002. A receptor for the heterodimeric cytokine IL-23 is composed of IL-12Rbeta1 and a novel cytokine receptor subunit, IL-23R.J. Immunol. 168:5699–5708 [DOI] [PubMed] [Google Scholar]
- Wilson N.J., Boniface K., Chan J.R., McKenzie B.S., Blumenschein W.M., Mattson J.D., Basham B., Smith K., Chen T., Morel F., et al. 2007. Development, cytokine profile and function of human interleukin 17-producing helper T cells.Nat. Immunol. 8:950–957 [DOI] [PubMed] [Google Scholar]
- Annunziato F., Cosmi L., Santarlasci V., Maggi L., Liotta F., Mazzinghi B., Parente E., Fili L., Ferri S., Frosali F., et al. 2007. Phenotypic and functional features of human Th17 cells.J. Exp. Med. 204:1849–1861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pene J., Chevalier S., Preisser L., Venereau E., Guilleux M.H., Ghannam S., Moles J.P., Danger Y., Ravon E., Lesaux S., et al. 2008. Chronically inflamed human tissues are infiltrated by highly differentiated th17 lymphocytes.J. Immunol. 180:7423–7430 [DOI] [PubMed] [Google Scholar]
- Cosmi L., De Palma R., Santarlasci V., Maggi L., Capone M., Frosali F., Rodolico G., Querci V., Abbate G., Angeli R., et al. 2008. Human interleukin 17–producing cells originate from a CD161+CD4+ T cell precursor.J. Exp. Med. 205:1903–1916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanier L.L., Chang C., Phillips J.H. 1994. Human NKR-P1A. A disulfide-linked homodimer of the C-type lectin superfamily expressed by a subset of NK and T lymphocytes.J. Immunol. 153:2417–2428 [PubMed] [Google Scholar]
- Huarte E., Cubillos-Ruiz J.R., Nesbeth Y.C., Scarlett U.K., Martinez D.G., Engle X.A., Rigby W.F., Pioli P.A., Guyre P.M., Conejo-Garcia J.R. 2008. PILAR is a novel modulator of human T-cell expansion.Blood. 112:1259–1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Keeffe J., Doherty D.G., Kenna T., Sheahan K., O'Donoghue D.P., Hyland J.M., O'Farrelly C. 2004. Diverse populations of T cells with NK cell receptors accumulate in the human intestine in health and in colorectal cancer.Eur. J. Immunol. 34:2110–2119 [DOI] [PubMed] [Google Scholar]
- Takahashi T., Dejbakhsh-Jones S., Strober S. 2006. Expression of CD161 (NKR-P1A) defines subsets of human CD4 and CD8 T cells with different functional activities.J. Immunol. 176:211–216 [DOI] [PubMed] [Google Scholar]
- Warrington K.J., Takemura S., Goronzy J.J., Weyand C.M. 2001. CD4+,CD28− T cells in rheumatoid arthritis patients combine features of the innate and adaptive immune systems.Arthritis Rheum. 44:13–20 [DOI] [PubMed] [Google Scholar]
- Vissers W.H., Arndtz C.H., Muys L., Van Erp P.E., de Jong E.M., van de Kerkhof P.C. 2004. Memory effector (CD45RO+) and cytotoxic (CD8+) T cells appear early in the margin zone of spreading psoriatic lesions in contrast to cells expressing natural killer receptors, which appear late.Br. J. Dermatol. 150:852–859 [DOI] [PubMed] [Google Scholar]
- Barrett J.C., Hansoul S., Nicolae D.L., Cho J.H., Duerr R.H., Rioux J.D., Brant S.R., Silverberg M.S., Taylor K.D., Barmada M.M., et al. 2008. Genome-wide association defines more than 30 distinct susceptibility loci for Crohn's disease.Nat. Genet. 40:955–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowat A.M. 2003. Anatomical basis of tolerance and immunity to intestinal antigens.Nat. Rev. Immunol. 3:331–341 [DOI] [PubMed] [Google Scholar]
- Fuss I.J., Heller F., Boirivant M., Leon F., Yoshida M., Fichtner-Feigl S., Yang Z., Exley M., Kitani A., Blumberg R.S., et al. 2004. Nonclassical CD1d-restricted NK T cells that produce IL-13 characterize an atypical Th2 response in ulcerative colitis.J. Clin. Invest. 113:1490–1497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov I.I., McKenzie B.S., Zhou L., Tadokoro C.E., Lepelley A., Lafaille J.J., Cua D.J., Littman D.R. 2006. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells.Cell. 126:1121–1133 [DOI] [PubMed] [Google Scholar]
- van Beelen A.J., Zelinkova Z., Taanman-Kueter E.W., Muller F.J., Hommes D.W., Zaat S.A., Kapsenberg M.L., de Jong E.C. 2007. Stimulation of the intracellular bacterial sensor NOD2 programs dendritic cells to promote interleukin-17 production in human memory T cells.Immunity. 27:660–669 [DOI] [PubMed] [Google Scholar]
- Liao F., Rabin R.L., Smith C.S., Sharma G., Nutman T.B., Farber J.M. 1999. CC-chemokine receptor 6 is expressed on diverse memory subsets of T cells and determines responsiveness to macrophage inflammatory protein 3 alpha.J. Immunol. 162:186–194 [PubMed] [Google Scholar]
- Kwon J.H., Keates S., Bassani L., Mayer L.F., Keates A.C. 2002. Colonic epithelial cells are a major site of macrophage inflammatory protein 3alpha (MIP-3alpha) production in normal colon and inflammatory bowel disease.Gut. 51:818–826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acosta-Rodriguez E.V., Rivino L., Geginat J., Jarrossay D., Gattorno M., Lanzavecchia A., Sallusto F., Napolitani G. 2007. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells.Nat. Immunol. 8:639–646 [DOI] [PubMed] [Google Scholar]
- Johansson-Lindbom B., Svensson M., Wurbel M.A., Malissen B., Marquez G., Agace W. 2003. Selective generation of gut tropic T cells in gut-associated lymphoid tissue (GALT): requirement for GALT dendritic cells and adjuvant.J. Exp. Med. 198:963–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlin C., Berg E.L., Briskin M.J., Andrew D.P., Kilshaw P.J., Holzmann B., Weissman I.L., Hamann A., Butcher E.C. 1993. Alpha 4 beta 7 integrin mediates lymphocyte binding to the mucosal vascular addressin MAdCAM-1.Cell. 74:185–195 [DOI] [PubMed] [Google Scholar]
- Briskin M., Winsor-Hines D., Shyjan A., Cochran N., Bloom S., Wilson J., McEvoy L.M., Butcher E.C., Kassam N., Mackay C.R., et al. 1997. Human mucosal addressin cell adhesion molecule-1 is preferentially expressed in intestinal tract and associated lymphoid tissue.Am. J. Pathol. 151:97–110 [PMC free article] [PubMed] [Google Scholar]
- Rott L.S., Briskin M.J., Andrew D.P., Berg E.L., Butcher E.C. 1996. A fundamental subdivision of circulating lymphocytes defined by adhesion to mucosal addressin cell adhesion molecule-1. Comparison with vascular cell adhesion molecule-1 and correlation with beta 7 integrins and memory differentiation.J. Immunol. 156:3727–3736 [PubMed] [Google Scholar]
- Ouyang W., Kolls J.K., Zheng Y. 2008. The biological functions of T helper 17 cell effector cytokines in inflammation.Immunity. 28:454–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchroo V.K., Umetsu D.T., DeKruyff R.H., Freeman G.J. 2003. The TIM gene family: emerging roles in immunity and disease.Nat. Rev. Immunol. 3:454–462 [DOI] [PubMed] [Google Scholar]
- Tsuda H., Michimata T., Sakai M., Nagata K., Nakamura M., Saito S. 2001. A novel surface molecule of Th2- and Tc2-type cells, CRTH2 expression on human peripheral and decidual CD4+ and CD8+ T cells during the early stage of pregnancy.Clin. Exp. Immunol. 123:105–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull D.M., Bookman M.A. 1977. Isolation and functional characterization of human intestinal mucosal lymphoid cells.J. Clin. Invest. 59:966–974 [DOI] [PMC free article] [PubMed] [Google Scholar]